Educational aims and objectives
This article aims to show the dental team’s challenges of maintaining implants and involving patients in the home care process.
Expected outcomes
Implant Practice US subscribers can answer the CE questions by taking the quiz to earn 2 hours of CE from reading this article. Correctly answering the questions will demonstrate the reader can:
- Identify some causes of peri-implant diseases.
- Realize the similarities and distinctions between the dental implant and natural tooth to provide basic guidelines for maintaining the long-term health of the dental implant.
- Realize how hygiene treatment, especially in certain areas, is integral to maintaining the implant.
- Realize scheduling needs for a hygiene re-care schedule depending upon the reason for and timing of the implant.
- Realize some differences in the connection of natural teeth or implants to the surrounding tissues for assessment of periodontal health.
Dr. Gregori M. Kurtzman and Debbie Zafiropoulos discuss the aspects of implants that make maintaining oral hygiene so important.
Dr. Gregori M. Kurtzman, along with Debbie Zafiropoulos, discusses appropriate professional care and effective patient oral hygiene for maintenance of implants
Introduction
Implant usage has become a common treatment modality in dentistry as a predictable long-term replacement of single teeth or full arches demonstrating restoration success.1-3 As the number of patients selecting dental implants as a treatment option continues to grow, the dental team must accept the challenges of maintaining these sometimes complex restorations. Those maintenance challenges also involve the patients and their home care.
Proper monitoring and maintenance are essential to ensure the longevity of the dental implant and its associated restoration through a combination of appropriate professional care and effective patient oral hygiene.4,5 So, maintaining implants is a double-edged sword with the dental team and patient both contributing to its long-term success or failure.
The value of using conventional periodontal parameters to determine peri-implant health is not clearly evident in the literature.6-8 Therefore, it is paramount that the dental implant team understands the similarities and distinctions between the dental implant and natural tooth. Subsequently, by examining the similarities and differences between a natural tooth and a dental implant, basic guidelines can be provided for maintaining the long-term health of the dental implant.
Direct anchorage of alveolar bone to a dental implant body provides a foundation to support a prosthesis and transmits occlusal forces to the alveolar bone. This is the definition of osseointegration.9,10 With the common acceptance of dental implants today as a viable and routine treatment option for the restoration of a partially edentulous or fully edentulous mouth, the dental team is faced with maintaining and educating those patients.
Recently, the focus of implant dentistry has changed from obtaining osseointegration, which is highly predictable, to long-term maintenance and health of the peri-implant hard and soft tissues. This can be achieved through appropriate professional care, patient cooperation, and effective home care.11-14 Since frequently the tooth or teeth are lost due to a lack of patient maintenance, the goal is to prevent old habits from re-emerging that could lead to periodontal issues around the implant and its potential loss. Patients must accept the responsibility for being co-therapists in maintenance therapy, so the dental team essentially must screen the potential implant patient.
Peri-implant challenges
Peri-implant diseases are prevalent, and prevalence of peri-implantitis increases over time. These might not be highly associated since the instances are influenced by distinct variables, which can include changes in the patient’s systemic health, the patient’s medications, and changes in the patient’s home care or ability to maintain oral hygiene.15 Peri-implant disease affects a significant number of implants. It is important to understand the difficulties in diagnosis of these diseases and risk factors, so that they may be modified to reduce the potential for disease occurrence or progression. Diagnosis and treatment planning based on a risk-benefit analysis should be performed subsequent to a comprehensive medical, dental, head-and-neck, psychological, temporomandibular disorder, and radiographic examination.16
Convincing evidence exists that bacterial plaque not only leads to gingivitis and periodontitis,17,18 but also can induce the development of peri-implantitis.10 Thus, personal oral hygiene must begin at the time of dental implant placement and should be modified using various adjunctive aids for oral hygiene to effectively clean the altered morphology of the peri-implant region before, during, and after implant placement. For instance, interproximal brushes can penetrate up to 3 mm into a gingival sulcus or pocket and may effectively clean the peri-implant sulcus.11 In addition to mechanical plaque control, chlorine dioxide has been demonstrated to reduce both plaque, gingival indices, and bacterial counts in the oral cavity without the potential issues with other rinses.12
Hygiene with dental implants is so tedious and critical to their long-term success that the patient and dental professional must exercise considerable effort. During the maintenance visit, the dental professional should concentrate on the peri-implant tissue margin, implant body, prosthetic abutment to implant collar connection, and the prosthesis.13
Clinical inspection for signs of inflammation — i.e., bleeding on probing, exudate, mobility, probable pockets, and a radiographic evaluation of the peri-implant bony housing — still remains the standard mode for evaluating the long-term status of endosseous dental implants. For instance, successful and stable endosseous dental implants exhibit no mobility. However, if there is clinically perceptible mobility, then subsequent to radiographic evaluation of the implant and its surrounding bony housing, the abutment retaining screw14 and/or prosthetic abutment collar interface should be examined for looseness or breakage.
All these modes of clinical assessment are used routinely, except for periodontal probing around peri-implant tissues that appear to be in a state of good health. The baseline data and data from subsequent re-care visits should be recorded in the daily progress notes to properly assess the peri-implant status longitudinally.
Subsequent to a thorough intraoral examination, unless there is visual evidence of soft tissue changes, i.e., inflammation of peri-implant tissue with even slight attachment loss or mucositis, and routine probing of the peri-implant tissue should not be performed.
Usually during the first year subsequent to restoring dental implants, a 3-month re-care schedule should be implemented, especially if the patient lost teeth because of periodontal disease. However, if after 12 months, the patient’s implants are stable and peri-implant tissues are healthy, then a 4-6 month re-care regimen can be implemented.15 However, be cognizant of each patient’s level of home care effectiveness, systemic health, and periodontal status of the peri-implant tissue when determining these re-care intervals.
With dental implant patients, the dental professional must evaluate the prosthetic components for plaque, calculus, and the stability of the implant abutment. Radiographs of dental implants should be taken every 12 to 18 months during these maintenance visits.16,17 For dental implant restorations that are screw-retained, the dental professional needs to remove the prosthesis at least once a year to more easily assess the status of the peri-implant’s hard and soft tissues, the existence of acceptable mobility of the prosthetic components or the implant fixture itself, and the patient’s level of home care effectiveness.18 Remember that the presence of any symptoms of infection, radiographic evidence of peri-implant bone loss, and/or neuropathies may be indicative of an ailing or failing implant.19 Inflammation at the gingival marginal tissue may be the first indication of initiation of peri-implantitis even in the absence of bleeding. Yet the periodontal complex around healthy natural teeth and implants are not similar.
Implants versus natural teeth
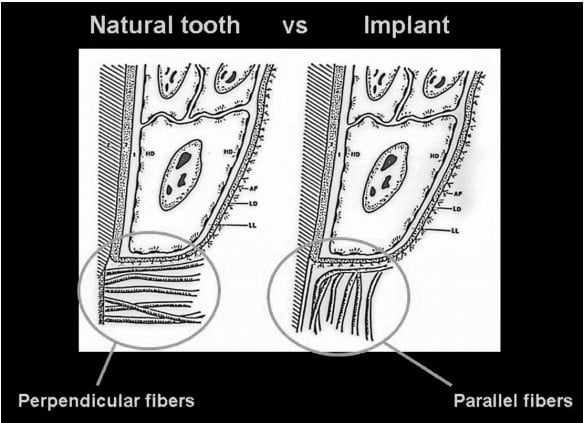
It is essential to understand the periodontal relationship between the gingiva and the structure it attaches to, whether it is a natural tooth or an implant (Figures 1 and 2). The fiber orientation of the gingival cuff around a natural tooth has those fibers attaching perpendicular to the long axis of the tooth (Figure 3). This fiber orientation acts as a barrier when a periodontal probe is inserted into the periodontal sulcus. The probe tip advances apically until the tip contacts the perpendicular fibers, resistance is felt, and the tip is halted from further apical progression without increased force on the instrument. This orientation is not found around implants. With an implant, the gingival fiber orientation is parallel to the implant’s long axis (Figure 4). When the periodontal probe is inserted into the periodontal sulcus around an implant, the probe tip advances, passing between the fibers of the gingival cuff until the crestal bone is contacted by the instrument’s tip, preventing it from further advancement (Figure 5). Due to the difficulty of defining a probing depth associated with peri-implant health, radiographic evaluation may be considered in the daily practice as a more predictable method for evaluation of the periodontal health of the implant.20-23
Recently, the focus of implant dentistry has changed from obtaining osseointegration, which is highly predictable, to longterm maintenance and health of the peri-implant hard and soft tissues. This can be achieved through appropriate professional care, patient cooperation, and effective home care



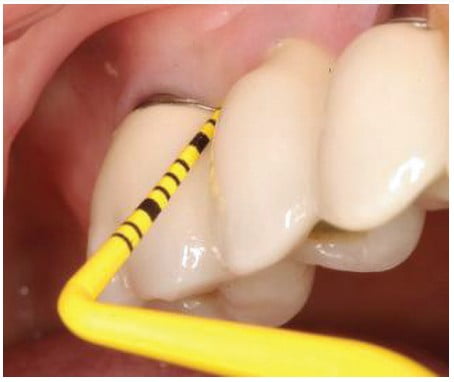
During re-care appointments, peri-implant periodontal probing should be performed where signs of infection are present — i.e., exudate, swelling, bleeding on probing, inflamed peri-implant soft tissue, and/or radiographic evidence of peri-implant alveolar bone loss. Lastly, periodontal probing of dental implants could damage the weak epithelial attachment around dental implants, possibly creating a pathway for the ingress of periodontal pathogens by inoculating them from saliva or biofilm deeper apically into the gingival tissue of the cuff.24,25 The use of probing depth and bleeding on probing assessments may lead to over-diagnosis and possibly to overtreatment of assumed biofilm-mediated peri-implantitis lesions.26 Due to the lack of a soft tissue physical stop to the tip of the probe related to fiber orientation, depth on probing will be higher around implants even when healthy tissue is present and should not assume that a probing of 6 or greater indicates a periodontal issue. Those probings can be used in comparison at future recall appointments to indicate if crestal bone has moved in an apical direction.
Commercially available plastic probes should be used when investigating the crevicular depth around dental implants that are presenting with signs of peri-mucositis (Figure 6). These plastic probes will not potentially damage the implant’s surface as a metal probe (stainless steel) may mar the surface creating biofilm accumulation points that may lead to peri-mucositis subgingivally (Figure 7). Titanium probes are available, but although they are less hard then stainless steel, may still damage the implant’s surface (Figure 8). The probing depth around dental implants may be related closely to the thickness and type of mucosa surrounding the implant. A healthy peri-implant sulcus has been reported to range from 1.3 mm to 3.8 mm, which is greater than those depths reported for natural teeth.27 In essence, the best indicator for evaluating an unhealthy site would be probing data gathered longitudinally.28,29 So, routine probing of dental implants is not predictable in identifying periodontal issues, especially in the absence of gingival inflammation being evident.


The peri-implant mucosal seal may be a less effective barrier to bacterial plaque than the periodontium around a natural tooth, tissue attachment.30-33 There is less vasculature in the gingival tissue surrounding dental implants compared to natural teeth. This reduced vascularity concomitant with parallel-oriented collagen fibers adjacent to the body of any dental implant make dental implants more vulnerable to bacterial insult.34 Peri-implant inflammation (mucositis) is a result of biofilm accumulation that disrupts the host-microbe homeostasis at the implant-mucosa interface. This results in an inflammatory lesion, but this is a reversible condition at the host level. The clinical implication is, therefore, that optimal biofilm removal is a prerequisite for prevention and management of peri-implant mucositis.24 With all of these reasons in mind, personal home care and consistent professional oral hygiene maintenance have proven to be critical to the success and longevity of dental implants. This is especially true in an environment with adjacent natural teeth, which if affected by periodontal disease, could act as a reservoir for pathogenic bacteria — i.e., gram-negative anaerobic rods — and seed the peri-implant sulcus.25-29
The physical characteristics of the peri-implant soft tissue are the focus of all patient oral hygiene instruction. The presence or absence of keratinized tissue in this critical area has not been unequivocally documented to state that peri-implant tissues are more vulnerable to the ingress of pathogenic bacteria with or without keratinized tissue being present around dental implants. However, the ability of the patient to maintain good oral hygiene around dental implants at home is facilitated by the presence of keratinized tissue surrounding the implants. Thus, if no keratinized tissue is around an implant, and if a pull from a frenum or a chronic peri-implant mucositis exists, then placement of a soft tissue autogenous or alloplastic connective tissue graft is recommended to facilitate proper mechanical oral hygiene maintenance.30-32 There is still no specific criteria for obtaining clinical data around dental implants that would allow proper monitoring, and detection of early possible failure of osseointegrated implants has not been clearly defined. Presently, the presence of mobility is the best indicator for diagnosis of implant failure. As opposed to natural teeth, dental implants exhibit minimal clinically undetectable movement because of the absence of a periodontal ligament.33,34 Therefore, healthy implants should appear nonmobile, even in the presence of peri-implant bone loss, if an adequate amount of supporting alveolar bone still exists.35,36
When monitoring the health of the peri-implant soft tissues, the practitioner should be cognizant of changes in soft tissue color, contour, and consistency. The presence of a fistulous tract could indicate the presence of a pathologic process or implant fracture. When noted in a partially edentulous arch, tracing the fistula tract with a gutta-percha cone inserted into the fistula prior to taking a radiograph will help determine if the infection resulting in the fistula is associated with the implant or the adjacent natural tooth.
Bleeding
There is controversy in the literature as to the accuracy and significance of bleeding upon probing around dental implants. Presently, the literature advocates the use of bleeding on probing as an indicator of peri-implant disease, as it may present prior to histologic signs of inflammation or concurrently with other signs of implant failure — i.e., bone loss. However, as previously mentioned, routine probing is not recommended. As stated, mobility is a clear sign of implant failure and may be present with or without the presence of bleeding.
Radiographic evaluation
Radiographic interpretation is one of the most useful clinical parameters for evaluating the status of an endosseous dental implant. Invasion of biologic width, predictable remodeling, or so-called saucerization, is an average marginal bone loss of 1.0 mm – 1.5 mm during the first year following prosthetic rehabilitation followed by an average of 0.2 mm of vertical bone loss every subsequent year.38,39 Thus, progressive bone loss around a dental implant that exceeds these averages may be indicative of an ailing or failing implant. Lastly, during radiographic evaluation, no evidence of a peri-implant radiolucency should be found, because such a rarefaction usually indicates infection or failure to osseointegration. Two-dimensional radiographs will not allow visualization of the buccal/facial and lingual crestal bone heights. 3D (CBCT) radiographic analysis may be required to properly evaluate bone levels when suspected or confirmed peri-implantitis is present, as the buccal/facial bone being less dense typically is resorbed prior to the interproximal bone.
Conclusion
Understanding the differences in the soft tissue attachment and the connection to the osseous bed housing the natural tooth or implant helps in maintaining those long-term. Natural teeth and implants, although providing identical functions allowing the patient to eat, have some differences in their connection to the surrounding tissues. Natural teeth have a fiber orientation at the gingival connection that acts as an effective barrier to bacteria in the biofilm from progressing apically, whereas the fiber orientation around implants does not provide that same bacterial restrictive factor. Routine periodontal probing of natural teeth as discussed can accurately identify periodontal disease related to that physical stop created by the perpendicular fibers at the base of the periodontal sulcus. Routine probing of implants is inaccurately related to the lack of a physical stop allowing the periodontal probe to progress through the tissue past the apical extent of the gingival cuff until a hard barrier, the crestal bone is encountered. This will give a false sense that periodontal issues are present around the implant, and appropriate treatment is needed to address that. Although mobility in a natural tooth can allow the tooth to function and be maintained unless it reaches pathologic levels, any mobility noted with an implant indicates it is failing and needs to be removed.
How those implants need to be maintained both by the dental practitioners (dentists and hygienists) and patients to ensure long-term survival will be addressed in part 2.
References
- Moraschini V, Poubel LA, Ferreira VF, Barboza Edos S. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: a systematic review. Int J Oral Maxillofac Surg. 2015;44(3):377-388.
- Malo P, de Araújo Nobre M, Lopes A, Moss SM, Molina GJ. A longitudinal study of the survival of All-on-4 implants in the mandible with up to 10 years of follow-up. J Am Dent Assoc. 2011;142(3):310-320.
- Maló P, de Araújo Nobre M, Lopes A, Ferro A, Botto J. The All-on-4 treatment concept for the rehabilitation of the completely edentulous mandible: A longitudinal study with 10 to 18 years of follow-up. Clin Implant Dent Relat Res. 2019;21(4):565-577.
- Orton GS, Steele DL, Wolinsky LE. The dental professional’s role in monitoring and maintenance of tissue-integrated prostheses. Int J Oral Maxillofac Implants. 1989;4(4):305-310.
- Kracher CM, Smith WS. Oral health maintenance dental implants. Dent Assist. 2010;79(2):27-36.
- Bauman GR, Mills M, Rapley JW, et al. Clinical parameters of evaluation during implant maintenance. Int J Oral Maxillofac Implants. 1992;7(2);220-227.
- Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42(suppl 16):S158-S171.
- Renvert S, Persson GR, Pirih FQ, Camargo PM. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J Periodontol. 2018;89(suppl 1):S304-S312.
- Rateischak KH, Wolf HF (eds ). Color Atlas of Dental Medicine: Implantology. Stuttgart, NY: Thieme Medical Publishers; 1995.
- Albrektsson T, Wennerberg A. On osseointegration in relation to implant surfaces. Clin Implant Dent Relat Res. 2019;21(suppl 1):4-7.
- Warrer K, Buser D, Lang NP, et al. Plaque-induced peri-implantitis in the presence or absence of keratinized mucosa: an experimental study in monkeys. Clin Oral implant Res. 1995;6(3):131-138.
- Kerémi B, Márta K, Farkas K, Czumbel LM, Tóth B, Szakács Z, Csupor D, Czimmer J, Rumbus Z, Révész P, Németh A, Gerber G, Hegyi P, Varga G. Effects of Chlorine Dioxide on Oral Hygiene – A Systematic Review and Meta-analysis. Curr Pharm Des. 2020;26(25):3015-3025. doi: 10.2174/1381612826666200515134450. PMID: 32410557.
- Balshi TJ. Hygiene maintenance procedures for patients treated with the tissue-integrated prothesis (osseointegration). Quintessence. 1986;17(2):95-102.
- Albrektsson T, Wennerberg A. On osseointegration in relation to implant surfaces. Clin Implant Dent Relat Res. 2019;21(suppl 1):4-7.
- Lee CT, Huang YW, Zhu L, Weltman R. Prevalences of peri-implantitis and peri-implant mucositis: systematic review and meta-analysis. J Dent. 2017;62:1-12.
- Meffert RM. Contemporary Implant Dentistry. Misch CE (ed). St. Louis, Mo: Mosby Year Book; 1993.
- Natto ZS, Almeganni N, Alnakeeb E, et al. Peri-Implantitis and Peri-Implant Mucositis Case Definitions in Dental Research: A Systematic Assessment. J Oral Implantol. 2019;45(2):127-131.
- Valm AM. The Structure of Dental Plaque Microbial Communities in the Transition from Health to Dental Caries and Periodontal Disease. J Mol Biol. 2019;431(16):2957-2969.
- Harvey JD. Periodontal Microbiology. Dent Clin North Am. 2017;61(2):253-269.
- Rösing CK, Fiorini T, Haas AN, et al. The impact of maintenance on peri-implant health. Braz Oral Res. 2019;33(suppl 1):e074.
- Weyant RJ. Characteristics associated with the loss and peri-implant tissue health of endosseous dental implants. Int J Oral Maxillofac Implants. 1994;9(1):95-102.
- Heitz-Mayfield LJA, Salvi GE. Peri-implant mucositis. J Periodontol. 2018;89(suppl 1):S257-S266.
- Nevins M, Langer B. The successful use of osseointegrated implants for the treatment of the recalcitrant periodontal patient. J Periodontol. 1995;66(2):150-157.
- Lang NP, Wetzel AC, Stich H, Caffesse RG. Histologic probe penetration in healthy and inflamed peri-implant tissues. Clin Oral Implants Res. 1994;5(4):191-201.
- Coli P, Sennerby L. Is Peri-Implant Probing Causing Over-Diagnosis and Over-Treatment of Dental Implants?. J Clin Med. 2019;8(8):1123.
- Coli P, Christiaens V, Sennerby L, Ruyn H. Reliability of periodontal diagnostic tools for monitoring peri-implant health and disease. Periodontol 2000. 2017;73(1):203-217.
- van Steenberghe D, Klinge B, Linden U, et al. Periodontal indices around natural and titanium abutments: a longitudinal multicenter study. J Periodontol. 1993;64(6):538-541.
- Karoussis IK, Müller S, Salvi GE, et al. Association between periodontal and peri-implant conditions: a 10-year prospective study. Clin Oral Implants Res. 2004;15(1):1-7.
- Mombelli A, Marxer M, Gaberthuel T, Grunder U, Lang NP. The microbiota of osseointegrated implants in patients with a history of periodontal disease. J Clin Periodontol. 1995;22(2):124-130.
- Artzi Z, Tal H, Moses O, Kozlovsky A. Mucosal considerations for osseointegrated implants. J Prosthet Dent. 1993;70(5):4274-4232.
- Thoma DS, Alshihri A, Fontolliet A, et al. Clinical and histologic evaluation of different approaches to gain keratinized tissue prior to implant placement in fully edentulous patients. Clin Oral Investig. 2018;22(5):2111-2119.
- Thoma DS, Naenni N, Figuero E, et al. Effects of soft tissue augmentation procedures on peri-implant health or disease: a systematic review and meta-analysis. Clin Oral Implants Res. 2018;29(suppl 15):32-49.
- Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants. (I). Success criteria and epidemiology. Eur J Oral Sci. 1998;106(1):527-551.
- Quirynen M, van Steenberghe D, Jacobs R, Schotte A, Darius P. The reliability of pocket probing around screw-type implants. Clin Oral Implants Res. 1991;2(4):186-192.
- Papaioannou W, Quirynen M, Nys M, van Steenberghe D.: The effect of periodontal parameters on the subgingival microbiota around implants. Clin Oral Implants Res. 1995;6(4):197-204.
- de Waal YC, Winkel EG, Meijer HJ, Raghoebar GM, van Winkelhoff AJ. Differences in peri-implant microflora between fully and partially edentulous patients: a systematic review. J Periodontol. 2014;85(1):68-82.
- Wang Q, Meng HX. Zhonghua Kou Qiang Yi Xue Za Zhi. [Research Progress in Microbiological Characteristics of Peri-Implant Disease] [Article in Chinese] 2017;52(12):773-776.
- Gholami H, Mericske-Stern R, Kessler-Liechti G, Katsoulis J. Radiographic bone level changes of implant-supported restorations in edentulous and partially dentate patients: 5-year results. Int J Oral Maxillofac Implants. 2014;29(4):898-904.
- Del Fabbro M, Ceresoli V. The fate of marginal bone around axial vs. tilted implants: a systematic review. Eur J Oral Implantol. 2014;7(suppl 2):S171-S189.
Stay Relevant With Implant Practice US
Join our email list for CE courses and webinars, articles and mores

 Gregori M. Kurtzman, DDS, MAGD, FPFA, FACD, FADI, DICOI, DADIA, is in private general practice in Silver Spring, Maryland. He is a former Assistant Clinical Professor at University of Maryland in the department of Restorative Dentistry and Endodontics and a former AAID Implant Maxi-Course assistant program director at Howard University College of Dentistry. He has lectured internationally on the topics of restorative dentistry, endodontics, implant surgery and prosthetics, removable and fixed prosthetics, and periodontics. Dr. Kurtzman has over 750 published articles globally. He has earned Fellowship in the AGD, ACD, ICOI, Pierre Fauchard, ADI, Mastership in the AGD and ICOI, and Diplomate status in the ICOI and American Dental Implant Association (ADIA). A consultant and evaluator for multiple dental companies, Dr. Kurtzman has been honored to be included in the “Top Leaders in Continuing Education” by Dentistry Today annually since 2006. He can be reached at
Gregori M. Kurtzman, DDS, MAGD, FPFA, FACD, FADI, DICOI, DADIA, is in private general practice in Silver Spring, Maryland. He is a former Assistant Clinical Professor at University of Maryland in the department of Restorative Dentistry and Endodontics and a former AAID Implant Maxi-Course assistant program director at Howard University College of Dentistry. He has lectured internationally on the topics of restorative dentistry, endodontics, implant surgery and prosthetics, removable and fixed prosthetics, and periodontics. Dr. Kurtzman has over 750 published articles globally. He has earned Fellowship in the AGD, ACD, ICOI, Pierre Fauchard, ADI, Mastership in the AGD and ICOI, and Diplomate status in the ICOI and American Dental Implant Association (ADIA). A consultant and evaluator for multiple dental companies, Dr. Kurtzman has been honored to be included in the “Top Leaders in Continuing Education” by Dentistry Today annually since 2006. He can be reached at  Debbie Zafiropoulos, EFDA, RDH, is the CEO of the OralED Institute, a Partner in Education for the Wellness Dentistry Network, an instructor with MoradoASC, and certified GBT Trainer for EMS-NA. Zafiropoulos works with top corporate companies in health, creating and delivering live and online educational programs to a worldwide audience. As a sought-after key opinion leader and author, she is determined to deliver programs of forward motion in prevention, science, and technology. In 2016, Zafiropoulos was a recipient of the SUNSTAR Award of Distinction. In 2017, she was recognized as one of the Top 25 Women in Dentistry for her advances in research and prevention of HPV-related oral cancer.
Debbie Zafiropoulos, EFDA, RDH, is the CEO of the OralED Institute, a Partner in Education for the Wellness Dentistry Network, an instructor with MoradoASC, and certified GBT Trainer for EMS-NA. Zafiropoulos works with top corporate companies in health, creating and delivering live and online educational programs to a worldwide audience. As a sought-after key opinion leader and author, she is determined to deliver programs of forward motion in prevention, science, and technology. In 2016, Zafiropoulos was a recipient of the SUNSTAR Award of Distinction. In 2017, she was recognized as one of the Top 25 Women in Dentistry for her advances in research and prevention of HPV-related oral cancer.

