OsteoLife Biomedical introduces a new freeze-dried bone allograft material that provides superior handling and easy adaptability to any defect morphology.
Dr. Arun K. Garg restores a severely resorbed alveolar buccal plate via guided-bone regeneration (GBR)
Allogeneic bone is favored by many implant surgeons for its convenience and unlimited availability, obviating the need for a second surgical procedure to harvest bone. Bone allografts are obtained from human cadavers through tissue banks that screen, process, and store them under highly sterile conditions. Because they are freeze-dried, the risk of antigenicity or disease transmission associated with cadaver bone is significantly reduced. Freeze-dried bone allografts are osteoconductive and osteoinductive and have the capacity to stimulate bone growth in areas where resorption has occurred and implants are needed. A variety of configurations are commercially available, including particulates, blocks, putty, and strips designed for extraction socket grafting, ridge and sinus augmentation, bone augmentation around implants, and repair of bony defects.
Recently, a new freeze-dried bone allograft material was introduced by OsteoLife Biomedical in a form that provides superior handling and easy adaptability to any defect morphology. Allogeneic Bone Fluff™ is a novel allograft with a proprietary patented 100% freeze-dried bone allograft (FDBA) configuration. When dry, Fluff has the unique consistency of a cotton ball, making it extremely easy to pick up and deliver to the surgical site. When it is hydrated, Fluff handles like a putty and provides cohesion and volume without any of the extraneous carriers that are added to other putty materials commonly used in dentistry. The consistency holds together throughout the procedure without any breakdown or disintegration.
Fluff combines the enhanced regenerative capacity of FDBA while maximizing the exposure of growth factors to cortical bone. It can be hydrated with sterile saline, platelet-rich plasma, or the surgeon’s biologic solution of choice and yields highly predictable results. The following case demonstrates the unique properties of this innovative bone grafting material.
Case report
A 55-year-old woman who underwent extraction of her six maxillary anterior teeth 5 years earlier secondary to tooth decay presented with a request for a fixed restorative solution. Her medical history was unremarkable with the exception of hypertension, which was well controlled with medication.
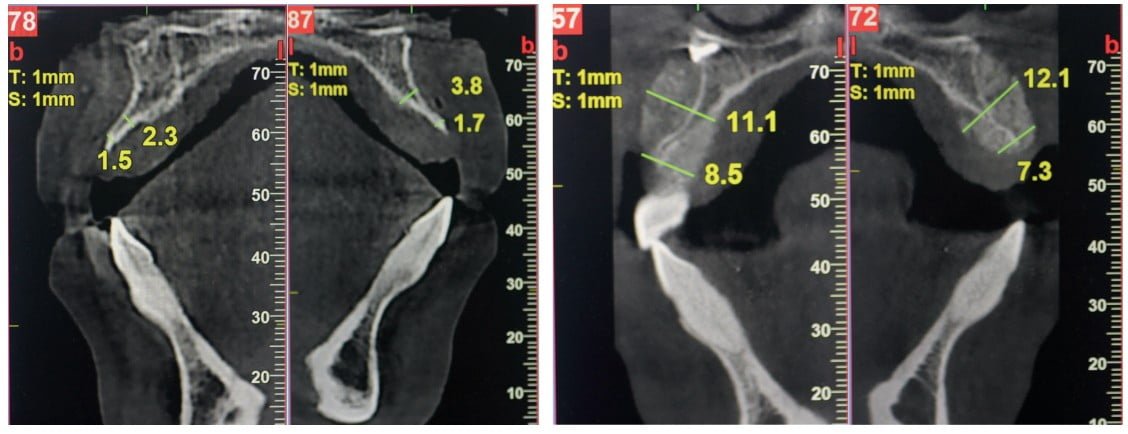
The clinical exam revealed deep concavities at the buccal aspect of the maxilla. Cone beam CT showed minimal residual cortical bone particularly in the cervical region, ranging from 0.5 mm to 3.8 mm of the available width. A treatment plan was developed to restore the severely resorbed alveolar buccal plate via guided-bone regeneration (GBR) using allogeneic Bone Fluff and cortical bone particles hydrated with PRP and covered with a slow-resorbing collagen membrane. After a 6-month healing period, two implants would be placed in the maxillary canine positions, followed by 4 months of healing, leading to delivery of a six-unit fixed anterior prosthesis. The plan was accepted by the patient who then provided her written informed consent.
Following local anesthesia of the buccal and palatal maxilla using 2% xylocaine with 1:100,000 epinephrine, a 15C blade was used to make a midcrestal incision from the mesial of the left first premolar to the mesial of the right lateral incisor. Intrasulcular incisions were made around the left and right and premolars along with release incisions at the base of the papillae of the second premolars. A full-thickness flap was elevated buccally and palatally to expose the anterior maxilla from the piriform rim to the apex of the palatal plate. Debridement of the graft site with a Molt curette and a No. 8 diamond bur under saline irrigation was followed by decortication of the buccal plate at the apical level of the bone with a No. 701 carbide bur.

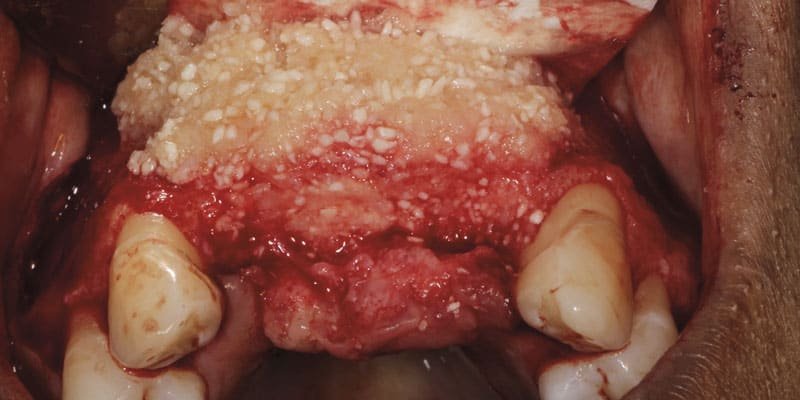
Meanwhile, cortical allogeneic Bone Fluff, which comes in the physical form of a cylinder (Figure 1), was sectioned into small pieces and hydrated with PRP to achieve a fluffy cotton ball-like consistency and combined with a particulated cortical bone allograft. Once activated, the composite graft was applied to the vertically deficient buccal ridge (Figure 2) and then covered with a collagen membrane that had been soaked in liquid PRP to improve its handling and increase the number of growth factors at the graft site. The membrane was carefully adapted under the palatal flap and fixated with two screws. The flap was repositioned, and the site was closed with multiple single interrupted sutures and one horizontal mattress suture using 5-0 monofilament nylon suture material. Postoperative CBCT slices showed an immediate increase in the width of the ridge in the cervical region from 7.3 mm to 8.5 mm and in the central area from 11.1 mm to 12.1 mm (Figure 3).
Allogeneic Bone Fluff™ is packaged in quantities of 1cc and 3cc and can be ordered direct from OsteoLife Biomedical (www.osteolifebiomedical.com).
After reading about OsteoLife Biomedical and its new allograft material, check out this article by Drs. John Lupovici and Robert Raimondi on staged extraction and guided bone regeneration before implant placement. https://implantpracticeus.com/staged-extraction-and-guided-bone-regeneration-gbr-before-implant-placement/
Stay Relevant With Implant Practice US
Join our email list for CE courses and webinars, articles and mores



