Prepared for today and the future of implant dentistry
MIS Implants Technologies has made its mark in the dental implant industry. Its humble beginnings laid down the strong foundation for MIS to become one of the leading global brands with innovative implant systems and related products. To understand how this has been achieved, it is important to understand the company’s philosophy and history.
The first MIS implants were manufactured in 1995 in a small industrial park in northern Israel. They were able to use that original building for many years, but as their distributors and reach grew around the globe, they planned and built a far larger facility. With distributors in Europe, South and Central America, the United States, and Canada, the Israel corporate home is where everything still begins, and activities are coordinated with those 60-plus distributors and subsidiaries. This new building itself houses hundreds of employees — from research and development, manufacturing, and quality assurance to warehousing, marketing, and the executive offices.
The mission of MIS Implants Technologies is to simplify the practice of dental implant-ology. Doctors around the world recognize that the design of the MIS implants and surgical kits accomplish just that. MIS is dedicated to offering quality and service to its clients. This does not mean only in the design and manufacturing phases of the firm’s iproducts, but also in the type of relationships its distributors build with individual clients. MIS considers itself to be agile and innovative, responding to the needs of practitioners and moving forward with new products based on scientific research. The company’s implant systems meet the needs of almost every dental implantologist and restorative doctor by offering different connections, body shapes, and thread designs. The evolution of their designs keeps up with current research and will continue to change as the practice of implantology evolves. From superior raw materials to packaging (the majority of the company’s implant systems are packaged with the appropriate final drill), MIS tries to simplify and improve the outcomes of dental implantology. To be more of a partner with the clinician, the MIS team launches additional products that serve to enhance the practice of dental implantology. It is this combination of core values that has allowed this company to grow steadily year after year.
Mr. Idan Kleifeld holds the CEO position at MIS Implants Technologies Ltd. in Israel. He has an impressive background with other manufacturing companies, all of which allowed him to transfer easily to MIS. His vision has helped MIS Implants to evolve into one of the world’s largest producers of dental implants.
 Mr. Doron Peretz has been part of MIS since those early days. He currently holds the title of Senior VP of Sales and Marketing. He has successfully expanded the role of MIS Implants to be more than just a manufacturer of dental implants. He has been a champion of expanding product lines and services from innovative bone products such as
Mr. Doron Peretz has been part of MIS since those early days. He currently holds the title of Senior VP of Sales and Marketing. He has successfully expanded the role of MIS Implants to be more than just a manufacturer of dental implants. He has been a champion of expanding product lines and services from innovative bone products such as
BondBone to two successful world conferences held in Cancun, Mexico, and Cannes, France. Each of these conferences has brought experts in the field of implantology to fabulous locations for lectures by experts in their respective specialties, hands-on workshops, and camaraderie among the attendees.
Along with the rest of the management team in Israel, MIS leaders have set their sights high and continue the forward momentum of the company. They know that the people they hire make a big difference between MIS and other implant companies. The MIS employees do truly work as a team, even internationally, to deliver the best product and service possible for their clients.
Newer entries into the market include the MCENTER with its MGUIDE guided surgical products and CAD/CAM 360 line of products, which feature custom-milled solutions for implant dentistry.
There are MCENTERs located in the United States, Germany, and Israel. The entire concept of the MGUIDE differs from conventional guided surgery applications in many ways. To start out with, the surgeon does not purchase the software. Each case is planned with the doctor once the MCENTER specialists have received the models (either digital or poured stone) and an STL file from CT imaging. A wax-up is developed for each arch, and the case is then planned from the desired outcome backward. This “top-down” planning helps to assure the end result is what the patient and clinician desire. The case is charged out separately or as part of a multi-case package.
In the last few months, MIS has announced a new implantology training series for doctors, which will be held at the corporate headquarters in Israel. The first multiple-day course, which will be given in English, will be held in February 2015. The curriculum will include (but is not limited to) treatment planning, CT evaluation and anatomical considerations, soft tissue management around implants, surgical complications, and live surgery observation. This will give clinicians from all over the world an opportunity to see, firsthand, the operations of this organization and to get a better understanding of what makes the MIS team so successful.
The MGUIDE stent and surgical kit also have unique features. The most impressive is the lack of keys (or “spoons”) to hold the guide in place. The MGUIDE surgical stent is designed to clip onto the undercuts of teeth to stay secure during the preparation of the osteotomy. Metal sleeves in the stent, along with the specially designed drills, determine the depth to which the osteotomy is prepared. The other immediately noticeable difference is the open framework of the stent, which allows for efficient irrigation and improved visibility over traditional stents. Imagine the increased confidence that a stent like this offers.
At the helm of the U.S. subsidiary is Mr. Motti Weisman who has held the position of CEO at MIS USA since its inception more than 12 years ago. He has helped to build the MIS brand through his dedication to the dental community. With almost 50 reps throughout the U.S., he has been able to provide excellent customer service directly to the dental community. With almost equal numbers of support personnel in the home office, they strive to be true partners to the doctors with whom they interact every day.
Mr. Weisman says, “We are in the middle of a digital revolution in dentistry that will change and affect all aspects of the science and art of dentistry.” The staff of the MCENTER in the U.S., along with assisting in the planning stage for guided surgery cases, also manufactures the stents in-house in state-of-the-art 3D printers. Each stent is subject to a rigorous quality assurance protocol prior to being sent to the surgeon. In addition to manufacturing the stents, the 3D printers are also currently utilized to print digital models for MGUIDE case planning.
Another recent addition to the U.S. capabilities is the milling center, which doctors and labs can utilize to make custom abutments with a pre-milled MIS interface as well as hybrid zirconia abutments. The CAD/CAM milling machines are specific to the material being milled and highly efficient at producing high-quality end products.
For education related to dental implantology, the Dental Implant Training Center (ditcusa.com) offers a variety of courses offering CE credits to doctors and staff members. Courses range from a basic introduction course to advanced bone grafts and sinus lift procedures.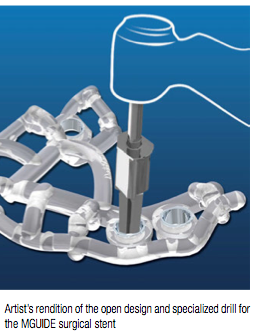
The newly launched MIS e-commerce site in the U.S. allows doctors to order their products online whenever they choose. This secure shopping site has been carefully built to make online ordering as simple as possible. The online store is well categorized with a strong search engine. Each product has excellent images with a magnifying feature available to zoom in for close-up viewing. Every order is reviewed to check on the compatibility of the products ordered to help avoid the shipment of unwanted products.
MIS USA has a strong presence at national specialty organization and local trade shows and is staffed by enthusiastic representatives who are always willing to speak to current or potential customers about the MIS products. As expected, the local representatives have experienced regional management to mentor and assist them. With the 20th anniversary year of MIS on the horizon, be ready for more innovative ideas and events to hit the implant industry.
Stay Relevant With Implant Practice US
Join our email list for CE courses and webinars, articles and mores

 Prepared for today and the future of implant dentistry
Prepared for today and the future of implant dentistry
