Dentsply Sirona proudly celebrates the three-year milestone of its first-ever company-initiated clinical registry, developed in collaboration with the McGuire Institute (iMc).
Launched in September 2021, this initiative has set a new standard in real-world clinical data collection, reinforcing Dentsply Sirona’s commitment to advancing dental care through innovation and evidence-based solutions. With more than 300 clinicians actively contributing to almost 2,000 implants placed across the US, the registry demonstrates extraordinary survival rates of the PrimeTaper EV Implant.
Charlotte, N.C., October 4, 2024. Dentsply Sirona and the McGuire Institute (iMc), a not for profit practice-based research network of periodontists and oral surgeons spanning the United States, launched the PrimeTaper EV Implant Registry with the aim of evaluating its performance in diverse real-world clinical settings. With more than 300 clinicians from across the U.S. contributing data on almost 2,000 implants, the registry has revealed an impressive 99% survival rate to date for the PrimeTaper EV Implant.
PrimeTaper EV Implant demonstrates high clinical reliability
The registry has become an invaluable resource, offering critical insights into the implant’s performance across a range of practice environments. Study participants encompass a broad spectrum of clinicians, from general practitioners to specialists, including both recent graduates and seasoned professionals. The patient demographics are equally varied, encompassing those with unremarkable medical histories as well as those with significant comorbidities. Yet, despite this diverse environment, participants in the study report outstanding results, with 96% of clinicians rating their experience with the PrimeTaper EV during surgical placement as either "good" or "very good," according to the top two categories of a Handling Survey questionnaire. This feedback reflects the implant’s high reliability, ease of use, and versatility in handling various bone densities, contributing to efficient procedures and optimal patient outcomes.
“Research is critical to the development of our next-generation solutions.”, said Tony Susino, GVP Implant & Prosthetic Solutions at Dentsply Sirona. “The real-world evidence obtained through this clinical registry provides valuable insights into the day-to-day experience of clinicians, helping us better understand how we can continue to meet their needs and ultimately advance dentistry – for the benefit of clinicians and patients alike.”
PrimeTaper EV Implant continues to transform digital implant dentistry
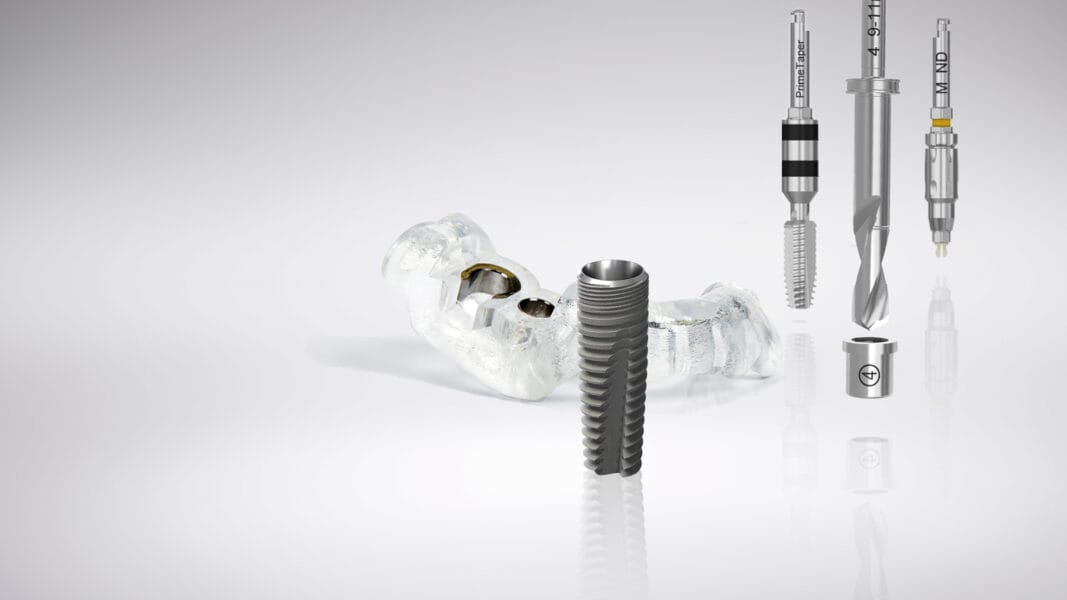
PrimeTaper EV Implant is a modern, fully tapered, self-tapping implant inspired by the proven Astra Tech Implant EV. Designed for efficient placement, predictable implant installation, and secure placement across varying bone densities. Implant clinicians get an implant with efficient handling, lasting bone care, enviable esthetics, and seamless integration into digital workflows.
“PrimeTaper EV Implant has great handling with its consistent torque build,” said Todd Scheyer, DDS from Houston, Texas “The taper and unique thread design make immediate installation easy and it fits in well with our digital workflow, making it more efficient. With the key features of Astra Tech Implant EV included in this modern implant, such as the OsseoSpeed surface and EV connection, it is a joy to have PrimeTaper Implant System as part of our practice and a benefit to our patients.”
The real-world evidence gathered in this study is another example of Dentsply Sirona driving toward its mission of transforming dentistry to improve oral health globally. For more information about PrimeTaper EV Implant please visit DS Primetaper | Dentsply Sirona USA.
About Dentsply Sirona
Dentsply Sirona is the world’s largest manufacturer of professional dental products and technologies, with over a century of innovation and service to the dental industry and patients worldwide. Dentsply Sirona develops, manufactures, and markets comprehensive solutions offering including dental and oral health products as well as other consumable medical devices under a strong portfolio of world class brands.
Dentsply Sirona’s products provide innovative, high-quality and effective solutions to advance patient care and deliver better and safer dental care. Dentsply Sirona’s headquarter is located in Charlotte, North Carolina. The company’s shares are listed in the United States on NASDAQ under the symbol XRAY. Visit www.dentsplysirona.com for more info.
Stay Relevant With Implant Practice US
Join our email list for CE courses and webinars, articles and mores



