Drs. A. Kuritsyn and M. Myroshnychenko treat a patient with SigmaGraft and autogenous bone chips. Read about this patient’s case and improvement in primary stability and bone quality.
Drs. A. Kuritsyn and M. Myroshnychenko, along with I. Borzenkova, discuss a clinical case with histological examination of obtained graft
Purpose
This case provides a histological examination of clinical cases demonstrating successful results during GBR. The main advantage of this approach is creation of the necessary osseous contour using an autogenous-derived round piece of bone block to hold and protect the augmentation. A combination of a mixture of InterOss® bovine bone (SigmaGraft) with autogenous bone chips in a 50-50 ratio followed by isolation with a resorbable collagen membrane (InterCollagen® Guide, SigmaGraft) makes it possible to obtain a high-quality augmentation. The results of the microscopic and morphometric examination of the biopsy specimen demonstrated the formation of a qualitative osteo-regeneration, proving the high efficiency of the treatment technique. This approach allows implants to be placed with sufficient primary stability and proven bone quality 4 months following GBR.
Abstract
In most clinical situations for a successful and predictable treatment with dental implants, it is necessary to create an optimal volume of alveolar ridge. To accomplish this at the first stage or with immediate implant placement, bone grafting is performed in a horizontal, vertical direction. Guided bone regeneration (GBR) has been used in dentistry for over 30 years.1,15,17
Materials and methods
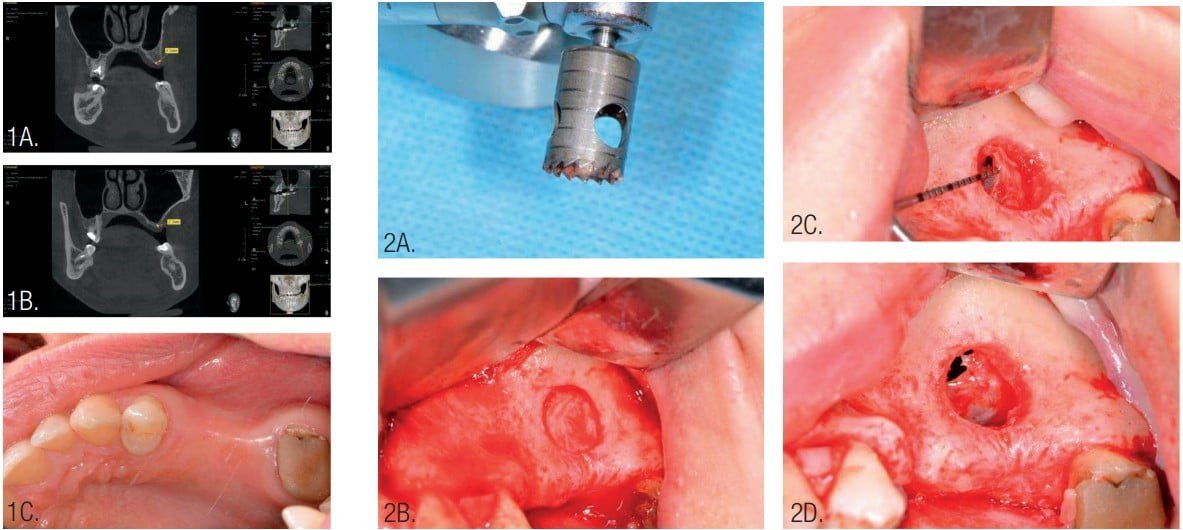
The 37-year-old female patient is a nonsmoker without any bad habits and general pathologies. The volume of bone in the area of the missing teeth (upper left second premolar and first molar) was insufficient for placement of dental implants with a long-term guaranteed result (Figure 1).
It was necessary to provide vertical bone grafting in the area of the maxillary sinus (sinus augmentation) and horizontal bone grafting in the area of the vestibular osseous defect with implant placement after 4 months of graft healing. CBCT controls were provided before and after each step of surgeries.
Periodontal cleaning was provided for the patient and rinsing with chlorhexidine 0.2% before surgery. The operation was performed under local infiltration anesthesia (Ultracain® D-S forte, Sanofi U.S., Bridgewater, New Jersey) and intravenous sedation. A trephine with an external diameter of 9 mm was used along with piezosurgery for access to the sinus for sinus membrane elevation.
The Schneiderian membrane of the maxillary sinus was elevated using curettes for further bone grafting. A perforation of Schneiderian membrane was identified and closed with resorbable suture material (Resorba® Resolon™ 6-0 Osteogenics Biomedical, Lubbock, Texas) (Figure 2).
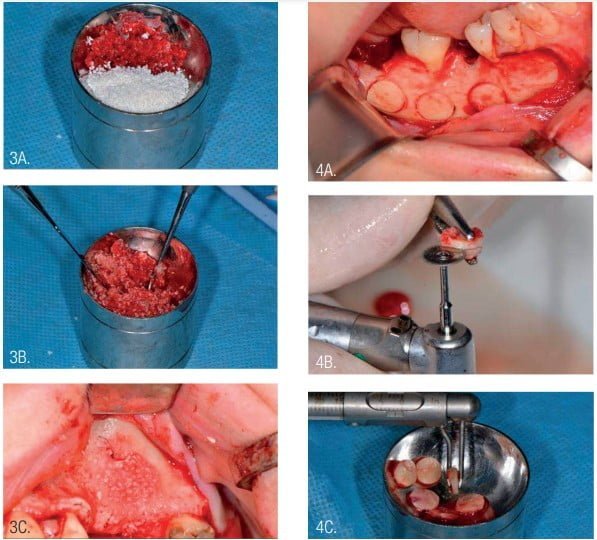
InterOss bovine bone (size 0.25 mm-1 mm) was mixed with autogenous bone chips in a 50-50 ratio graft and placed into the subantral sinus space for vertical bone graft.3,4,6,7 Autogenous bone chips were collected with a safe bone scraper (Geistlich Pharma, Princeton, New Jersey) from retromolar area of the mandible (Figure 3).
The round autogenous blocks were harvested with a trephine from the line oblique external of the mandible. Splitting of the round block was accomplished with a diamond disk. The approximate width of round lamina (puck) was 1.5 mm-2 mm (Figure 4).
The access to the sinus was closed with a round bone lamina (puck). The external diameter of trephine during access to the sinus was 9 mm, the same as the internal diameter of the puck. However, the puck was suitable for sinus closure (Figure 5).
The next surgical step was formation of a new bony contour in the area of the horizontal defect. Round bone plates (pucks) were used for GBR. Pucks were fixed with microscrews (Jeil Medical Corporation, Seoul, Republic of Korea) at the required distance from the jaw, depending on the need to repair the defect (Figure 6).
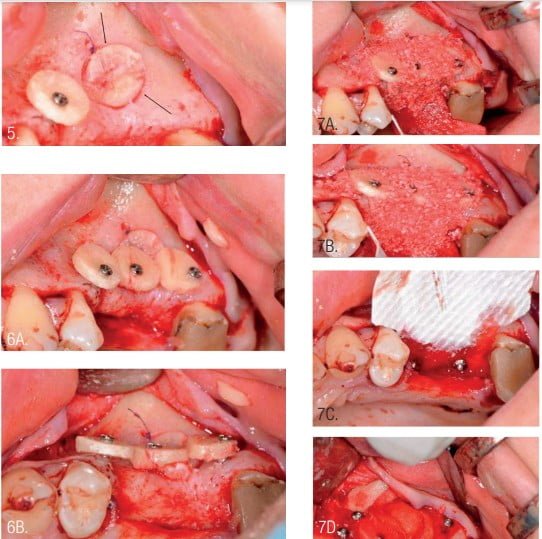
Filling of the defect around and under the installed round bone plates was performed using a mix xenograft (InterOss) and autogenous bone chips in the same ratio and previously mixed (Figure 3). The bone-grafted area was covered with a resorbable collagen membrane (InterCollagen Guide, size 30 x 40 mm) which was fixated with titanium pins (Figure 7).5,8,9,12,13
The main advantage of the collagen membrane is its ability to stretch. The membrane isolates the graft from soft tissue and maintains the shape of the new bony contour. The difference with the approach presented from Urban’s Sausage technique5 is that the new bony contour is supported by the round bone plates. This protects the area from muscle activity and chewing function and maintains a given shape during the healing phase of treatment.3,13,16
The site is closed in two steps using mattress sutures and interrupted sutures without tension utilizing resorbable sutures (Resorba®Glycolon™ 6.0, Osteogenics) and Resorba Resolon 5.0 sutures. The sutures were removed after 14 days.
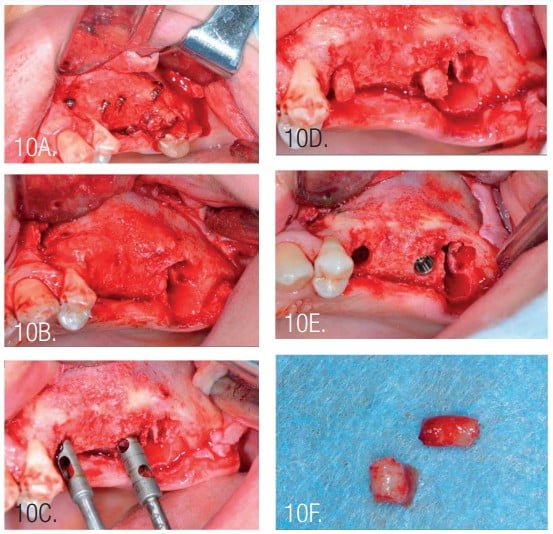
Postoperative antibiotic administration was performed with amoxicillin for 10 days at 2*1g per day. Following healing, the next surgery was performed 4 months post osseous grafting (Figure 8). After flap elevation, the screws and pins were removed, and implants were placed. Implants were NeoBiotech IS III, 4.0 mm x 10 mm (Pasadena, California) in the second premolar area and a 4.5 mm x 10 mm implant at the first molar. The second molar was extracted during second surgery as it was deemed of poor prognosis.
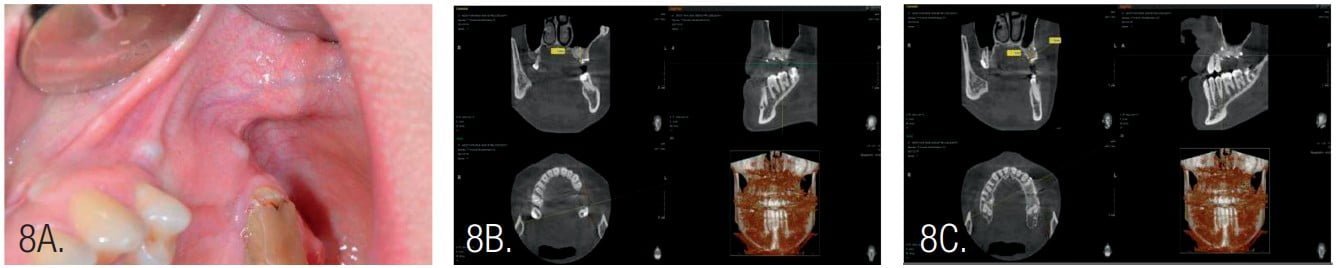
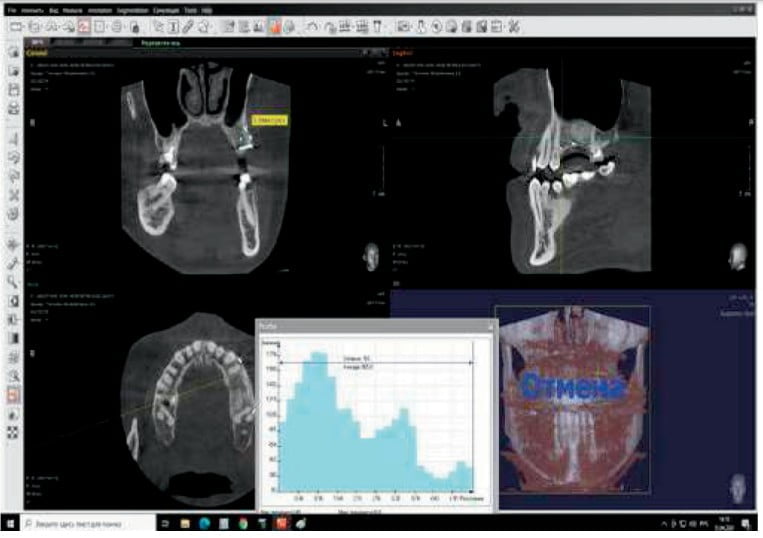
The width of the augmented bone was measured following site exposure, and volume and density of bone was checked with CBCT before implant placement. Osteotomy preparation was initiated using the trephine (diameter 2.5/3.5). This allowed removal of bone cores for histological examination (Figure 10).
Histological examination
The resulting bone material was placed into a 10% solution of neutral formalin (pH 7.4) for 24 to 48 hours, decalcified, following generally accepted technique, and embedded in paraffin. From the paraffin blocks, serial sections with a thickness of 4-5 mm were made, stained with a hematoxylin and eosin picrofuchsin solution according to van Gieson. The slides were studied using an Olympus BX-41 microscope (Japan).
The implants were exposed 2 months after implant placement with soft tissue management. A full gingival graft (FGG) from the palate was used for creation of sufficient keratinized tissue.
Results
- CBCT examination before bone graft and 4 months after: In the area of the missing second premolar, a measuring of bone showed a height of 11 mm and width of 4.6 mm before surgery. Following surgery, the resulting height was 13 mm and width of 9.6 mm.
- Average density of the grafted area was 905 (Hounsfield units).
- Primary stability during inserting of the implants was acceptable with insertion torque of 35 Ncm at the second premolar and 25 Ncm at the first molar site.
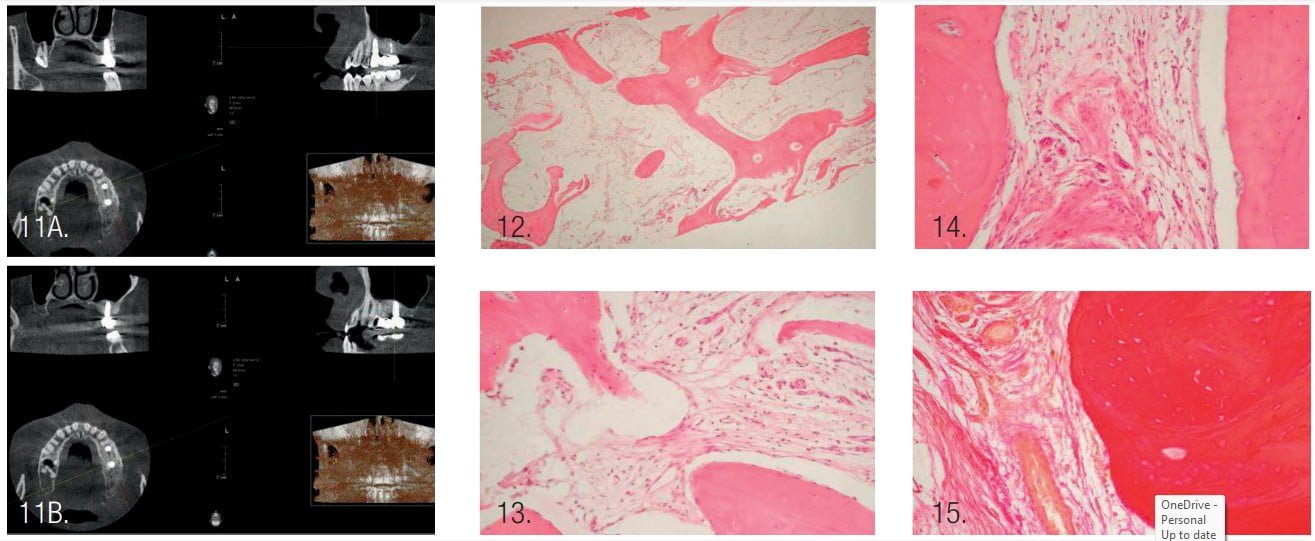
- CBCT 1 year after prosthetic placement showed a stable level of bone tissue around the implants, absence of inflammation in the maxillary sinus, and bone tissue of the alveolar process (Figure 11).
Results of histological examination
Observational microscopy revealed osteo-regeneration, characterized by the presence of bone trabeculae, with connective and adipose tissues between them. The average value of the specific volume of bone trabeculae was 65.9%, connective tissue — 22.5%, and adipose tissue — 11.6%.
The bone trabeculae were located chaotically and had different sizes. Some of the bone trabeculae formed anastomoses between themselves. The bone trabeculae were uniformly pink in color, which indicated good mineralization.
Osteocytes were found within the trabeculae. On the surface of some osteoid trabeculae, osteoblasts were identified that had a polygonal shape and sharply basophilic cytoplasm. A few large multinucleated osteoclasts were found on the periphery of some osteoid trabeculae.
Connective and adipose tissues with the presence of vessels and polymorphic cell infiltration — represented mainly by lymphocytes, histiocytes, macrophages, and few cells of fibroblast series — were determined between the bone trabeculae. Connective tissue fibers were thinned in some fields of view, thickened in other fields that had a pink color when stained with hematoxylin and eosin, or dark red when stained with picrofuchsin according to van Gieson.18 Adipocytes of adipose tissue, when stained with hematoxylin and eosin, looked like round-oval colorless voids with the clear boundaries. The nuclei in adipocytes were localized at the periphery of the cells.
Conclusion
The combination between xenograft and autogenous bone chips in a 50-50 ratio with round split blocks (pucks) allows achievement of enough volume and quality of bone for successful implantation. Round split blocks have a clear advantage for creating a new hard osseous ridge contour in comparison with PTFE membranes or titanium mesh. The results of the survey microscopy and morphometric examination of the biopsy specimen indicate the formation of high-quality osteo-regeneration, which proves the high efficiency of the treatment. This approach allows implants to be placed with sufficient primary stability and proven bone quality 4 months after GBR.
SigmaGraft can be an effective addition to your implant armamentarium. To read more about other approaches of augmenting hard tissues in implant dentistry, read “Understanding bone augmentation and regeneration.” Pass the quiz and receive two credits! https://implantpracticeus.com/ce-articles/understanding-bone-augmentation-and-regeneration/
- Buser D. 20 Years of Guided Bone Regeneration in Implant Dentistry (2nd edition). Quintessence Publishing: USA; 2009.
- Khoury F, Hanser T. Mandibular bone block harvesting from the retromolar region: a 10-year prospective clinical study. Int J Oral Maxillofac Implants. 2015;30( 3): 688-697.
- Khoury F, Hanser T. Three-dimensional vertical alveolar ridge augmentation in posterior maxilla: a 10-year clinical study. Int J Oral Maxillofac Implants. 2019; 34( 2) :471-480.
- Simion M, Fontana F, Rasperini G, Maiorana C. Long-term evaluation of osseointegrated implants placed in sites augmented with sinus floor elevation associated with vertical ridge augmentation: a retrospective study of consecutive implants with 1- to 7-year follow-up. Int J Periodontics Restorative Dent. 2004;24(3):208-221.
- Urban IA, Jovanovic SA, Lozada JL. Vertical ridge augmentation using guided bone regeneration (GBR) in three clinical scenarios prior to implant placement: a retrospective study of 35 patients 12 to 72 months after loading. Int J Oral Maxillofac Implants. 2009; 24(3):502-510.
- Fontana F, Santoro F, Maiorana C, et al. Clinical and histologic evaluation of allogeneic bone matrix versus autogenous bone chips associated with titanium-reinforced e-PTFE membrane for vertical ridge augmentation: a prospective pilot study. Int J Oral Maxillofac Implants. 2008;23(6):1003-1012.
- Lee EY, Kim ES, Kim KW. Vertical augmentation of maxillary posterior alveolar ridge using allogenic block bone graft and simultaneous maxillary sinus graft. Maxillofac Plast Reconstr Surg. 2019;36(5):224-229.
- Buser D, Witteneben J, Bornstein MM, et al. Stability of contour augmentation and asthetic outcomes of implant-supported single crowns in the esthetic zone: 3-year results of a prospective study with early implant placement post-extraction. J Periodontol. 2011;82(3):342-349.
- Kang DW, Yun PY, Choi YH, Kim YK. Sinus bone graft and simultaneous vertical ridge augmentation: case series study. Maxillofac Plast Reconst Surg. 2019;16(41):36.
- Urban IA, Montero E, Monje A, Sanz-Sánchez I. Effectiveness of vertical ridge augmentation interventions: A systematic review and meta-analysis. J Clin Periodontol. 46(Suppl 21):319-339.
- Khoury F, Happe A. Soft tissue management in oral implantology: A review of surgical techniques for shaping an esthetic and functional peri-implant soft tissue structure. Quintessence Int. 2000;31(7):483-499.
- De Stavola L, Tunkel J. Results of vertical bone augmentation with autogenous bone block grafts and the tunnel technique: A clinical prospective study of 10 consecutively treated patients. Int J Perio Restorative Dent. 2013;33(5):651-659.
- Khoury F, Tunkel J. Bone augmentation and soft tissue management. In: Khoury F, Antoun H, Missika P (eds.) Bone Augmentation in Oral Implantology. Berlin, London: Quintessence; 2006.
- Khoury F, Keller P, Keeve PL. Stability of grafted implant placement sites after sinus oo r elevation using a layering technique: 10-year clinical and radiographic results. Int J Oral Maxillofac Implants. 2017;32(5):1086-1096.
- Esposito M, Grusovin MG, Felice P, et al. Interventions for replacing missing teeth: horizontal and vertical bone augmentation techniques for dental implant treatment. Cochrane Database of Systematic Reviews.
- Felice P, Esposito M, Scarano A, et al. A comparison of two techniques to augment maxillary sinus with the lateral approach: no grafting procedure vs an organic bone placement. Preliminary histological and clinical outcomes of a randomized controlled clinical trial. Clinical oral Implants Research. 2009;20:973(Abs 261).
- Parma-Benfenati S, Tinti C, Albrektsson T, Johansson C. Histologic evaluation of guided vertical ridge augmentation around implants in humans. Int J Perio Restorative Dent. 1999:424-37.
- Khalid AL. Eponyms in the dermatology literature linked to stains used in skin biopsies. Our Dermatology Online. 2013; 4(4):569-572.
Stay Relevant With Implant Practice US
Join our email list for CE courses and webinars, articles and mores



