Accuracy assessment and surgeon feedback on Navident Dynamic Navigation System

Navident guides the surgeon to place implants as planned on a CBCT image volume similar to a GPS that guides drivers along a route planned on a map.
[userloggedin]
The system combines restoration-guided planning and surgical guidance in a single laptop and a stereoscopic pose tracking camera hovering over the patient’s chest. Being much simpler and faster than static guides, Navident allows the full workflow of CBCT scanning, planning, and guided implantation to be performed in a single, short patient appointment.
The system’s developer, ClaroNav Inc., headquartered in Toronto, Canada, has been successfully developing and marketing imaging and navigation solutions to the global medical community for over 15 years. Navident is the result of close collaboration between ClaroNav Inc. and the University of Toronto Faculty of Dentistry. The system has received CE Mark clearance in July of 2015 and has since seen rapidly rising sales in Europe and East Asia. At the time of the study reported here, FDA clearance
was pending.
The study
To assess the early impact of Navident in clinical usage, in March of 2016, ClaroNav contacted Navident users and requested that they participate in a Clinical Usage Study consisting of case reports, accuracy assessments, and surgeon feedback collected through detailed questionnaires on Navident’s impact on each case and an overall practice impact.

Seven surgeons practicing at sites located in Canada, Belgium, Italy, Sweden, and Colombia responded to ClaroNav’s request. Respondents’ implantation experience ranged from a recent graduate to placing 6,000 implants over 20 years. Collectively, by the time they responded, they have performed about 350 Navident-guided implantations in 150 patients. None of the surgeons received, or expected to receive, financial support from ClaroNav.
Twenty-one patient cases containing 36 implants were submitted. In instances where the clinicians provided post-surgical CT data, a validated plan-to-implant comparison program was used to obtain placement-error measurements.
Case reports and placement accuracy
No adverse events or complications were reported, and no major usability issues interfering with effective usage were identified.
Where post-implantation CT images were provided for cases, placement accuracy could be reliably measured by carefully registering the pre- and post-images and measuring the geometric deviation between the planned and actual positions of each implant. One surgeon submitted nine cases with 14 guided implantations, enabling statistically significant accuracy numbers to be computed. The results are shown in Table 1.
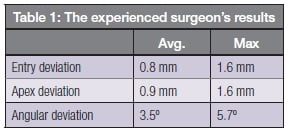 These accuracy results compare favorably with those obtained with static guides. For example, a systematic review of published static guide accuracy studies, including ones done on models or cadavers, published by Tahmaseb, et al., in 2014, reported on corresponding average/max of reported errors of 1.1/4.5 mm, 1.4/7.9 mm and 3.9/21°.
These accuracy results compare favorably with those obtained with static guides. For example, a systematic review of published static guide accuracy studies, including ones done on models or cadavers, published by Tahmaseb, et al., in 2014, reported on corresponding average/max of reported errors of 1.1/4.5 mm, 1.4/7.9 mm and 3.9/21°.
The remaining implantations were evaluated based on clinical and radiographic assessments and were deemed by all surgeons to be accurately placed.
Clinical usage feedback
The participating surgeons were asked to mark their degree of agreement with a set of statements related to the usage of the system. The combined results are tabulated in Table 2 with each star representing one response.
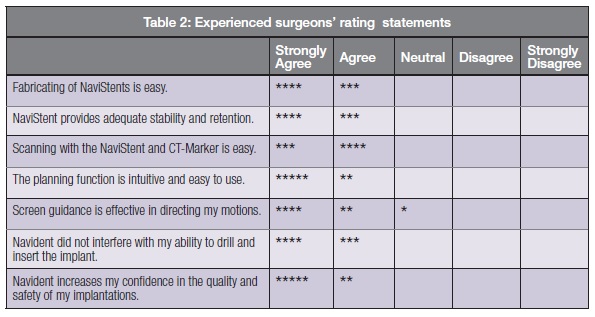
Of particular note is that the highly agreeable responses to the last statement indicate that Navident is having the intended benefit of increased confidence in the quality and safety of implantation for surgeons across a wide spectrum of experience and skill levels.
Participants were also asked to comment on their experiences with Navident in a free-form format. The majority of the comments praised the system’s design and clinical usefulness. Other statements proposed improvements to some aspects of the system design, but there were no repetitions, implying perhaps, that these issues are mostly a matter of personal preferences and the case mix of each surgeon.
One surgeon reported that in one case, navigation usage was aborted following a failed accuracy check. Subsequent investigation revealed that the inaccuracy was caused by modifications made by the surgeon, contrary to usage instructions to the jaw-tracking attachment, NaviStent, following the CBCT scan. This appears to indicate that, unlike with static guides, the accuracy of dynamic navigation systems can be assessed prior to performing any osteotomy, making them inherently safer to use.
Conclusions
The results of this survey provide strong evidence supporting the following claims:
- Navident is safe: Usage errors that may lead to incorrect guidance are rare and are caught by the accuracy check prior to drilling.
- Navident is effective: It boosts surgeon’s confidence in the quality and safety of the implantations and enables flapless surgery with accuracy matching or surpassing that of static guides.
- Navident is easy to use: Users are satisfied or very satisfied with all key aspects of Navident’s design.
The Navident is currently undergoing 510(k) premarket review by the FDA.
This information was provided by ClaroNav Inc.
[/userloggedin]
[userloggedout][/userloggedout]
Stay Relevant With Implant Practice US
Join our email list for CE courses and webinars, articles and mores


