Drs. Cemal Ucer and Eddie Scher summarize a recent series of papers to provide an evidence-based protocol for implant placement in the mandible
The trigeminal nerve (TGN) and its branches as well as the lingual artery are at risk during implant surgery in the mandible. Complications in this area are serious — even life threatening — and often result in medico-legal complaints.
This article aims to summarize the findings of a recent series of papers published by Yilmaz, Ucer, Scher, Suzuki, and Renton (2016; 2017), and Renton and Yilmaz (2011) and to outline a risk management protocol for implant placement surgery in the man-dible, based on these results and previous protocols published by Scher (2002) and Renton (2013).
Educational aims and objectives
This article aims to highlight the risk factors for nerve and blood vessel injury related to dental implants and to present an evidence-based protocol of risk management for placement of implants in the mandible.
Expected outcomes
Implant Practice US subscribers can answer the CE questions to earn 2 hours of CE from reading this article. Take the quiz here. Correctly answering the questions will demonstrate the reader can:
- Realize an evidence-based understanding of the cause of collateral damage to vital tissues related to implant surgery and an ability to apply risk management strategies when placing implants in the mandible.
- Identify a protocol of risk management strategies in mandibular implant surgery.
- Recognize the benefits of surgical guides.
- Recognize the importance of flap design.
- Identify some postoperative risk management strategies.
Nerve damage can be caused by direct or indirect mechanical, thermal, ischemic, chemical injury, or infectious means and can also occur following local anesthetic (LA) injections. There are concerns that the incidence of iatrogenic trigeminal nerve injuries (TGI) may be on the rise with the increasing popularity of implant treatment and the surgery being undertaken by clinicians of varying levels of experience and training.
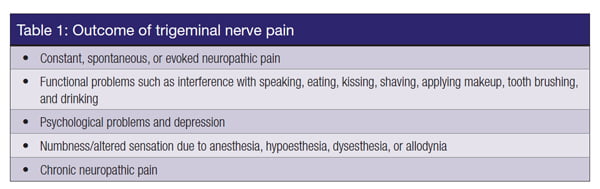 The resulting neurosensory disturbances (NSDs) are characterized by altered sensation or numbness affecting the area that is innervated by the branches of the TGN. More significantly, iatrogenic TNIs commonly cause chronic neuropathic pain resulting in constant interference with speaking, eating, kissing, shaving, applying makeup, tooth brushing, and drinking. These injuries have a significant negative effect on the patient’s self-image and quality of life with profound psychological effects (Renton and Yilmaz, 2011; Yilmaz, et al., 2016) (Table 1).
The resulting neurosensory disturbances (NSDs) are characterized by altered sensation or numbness affecting the area that is innervated by the branches of the TGN. More significantly, iatrogenic TNIs commonly cause chronic neuropathic pain resulting in constant interference with speaking, eating, kissing, shaving, applying makeup, tooth brushing, and drinking. These injuries have a significant negative effect on the patient’s self-image and quality of life with profound psychological effects (Renton and Yilmaz, 2011; Yilmaz, et al., 2016) (Table 1).
 Our research showed that specific training in the prevention and management of iatrogenic nerve damage related to dental implants might be lacking or inadequate during implant training. This deficiency should be addressed by course providers urgently given the devastating nature of this type of complication. Implant surgeons do not seem to be aware that iatrogenic nerve damage is a serious surgical complication that is reportable to the Care Quality Commission (CQC) in the UK (Yilmaz, et al., 2016; 2017)
Our research showed that specific training in the prevention and management of iatrogenic nerve damage related to dental implants might be lacking or inadequate during implant training. This deficiency should be addressed by course providers urgently given the devastating nature of this type of complication. Implant surgeons do not seem to be aware that iatrogenic nerve damage is a serious surgical complication that is reportable to the Care Quality Commission (CQC) in the UK (Yilmaz, et al., 2016; 2017)
 Furthermore, there is evidence to suggest that risk assessment, treatment planning, and the consent processes performed by implant dentists may be inadequate or short of the best-practice guidelines in implant dentistry. Implant organizations, including the International Team for Implantology (ITI), Academy of Osseointegration (AO), European Association for Osseointegration (EAO), International Congress of Oral Implantologists (ICOI), and so on have evidence-based guidelines and good-practice recommendations, but clinicians may not be using these recommendations fully — particularly when placing implants in the mandible.
Furthermore, there is evidence to suggest that risk assessment, treatment planning, and the consent processes performed by implant dentists may be inadequate or short of the best-practice guidelines in implant dentistry. Implant organizations, including the International Team for Implantology (ITI), Academy of Osseointegration (AO), European Association for Osseointegration (EAO), International Congress of Oral Implantologists (ICOI), and so on have evidence-based guidelines and good-practice recommendations, but clinicians may not be using these recommendations fully — particularly when placing implants in the mandible.
 The evidence shows that inaccurate radiological identification of the inferior alveolar nerve (IAN) or mental nerve (MN) and their anatomical variations are the most frequent cause of TGIs. Interestingly, disclosure of the relative risk of surgery and benefits of alternative implant treatment strategies was not deemed to be essential to an individualized consent process by a quarter of the participants (Yilmaz, et al., 2016).
The evidence shows that inaccurate radiological identification of the inferior alveolar nerve (IAN) or mental nerve (MN) and their anatomical variations are the most frequent cause of TGIs. Interestingly, disclosure of the relative risk of surgery and benefits of alternative implant treatment strategies was not deemed to be essential to an individualized consent process by a quarter of the participants (Yilmaz, et al., 2016).
 Nevertheless, the results showed that the incidence of TGI is reduced when implant surgery is carried out by experienced and well-trained surgeons using a standardized technique and certain preventive measures (Yilmaz, et al., 2016; Devine, et al., 2014). The authors therefore recommended that surgeons should acquire evidence-based specific skills in assessment and planning of implant surgery, use appropriate diagnostic imaging, and employ evidence-based strategies to minimize the collateral surgical damage when placing implants in the mandible.
Nevertheless, the results showed that the incidence of TGI is reduced when implant surgery is carried out by experienced and well-trained surgeons using a standardized technique and certain preventive measures (Yilmaz, et al., 2016; Devine, et al., 2014). The authors therefore recommended that surgeons should acquire evidence-based specific skills in assessment and planning of implant surgery, use appropriate diagnostic imaging, and employ evidence-based strategies to minimize the collateral surgical damage when placing implants in the mandible.
 The literature shows that cone beam computer tomography (CBCT) could play an important role in reducing the risk of TG damage and any associated morbidity. It is important to note that the American Academy of Oral and Maxillofacial Radiology (AAOMR), in its revised evidence-based position statement on the selection criteria for radiology in implant dentistry, recommends that CBCT should be used for the assessment of all dental implant sites (Tyndall, et al., 2012).
The literature shows that cone beam computer tomography (CBCT) could play an important role in reducing the risk of TG damage and any associated morbidity. It is important to note that the American Academy of Oral and Maxillofacial Radiology (AAOMR), in its revised evidence-based position statement on the selection criteria for radiology in implant dentistry, recommends that CBCT should be used for the assessment of all dental implant sites (Tyndall, et al., 2012).
In this respect, the use of small field of view (sFOV) CBCT scanning could be regarded as essential as a choice of diagnostic imaging in the mandible subject to fulfilling national justification criteria such as those published by the FGDP. This new protocol comprises a structured preoperative assessment, diagnosis, planning, and perioperative risk management strategies.
A protocol of risk management strategies in mandibular implant surgery
Iatrogenic nerve damage can occur if adequate clearance is not allowed when placing an implant near the mandibular branches of the TG nerve. It can also occur when the surgeon diverges from the planned implant position, either inadvertently or deliberately — for example, if unexpected anatomical variations are encountered during surgery (Table 2). A higher standard of care with meticulous planning and surgical precision is therefore essential.
TGI should be fully avoidable due to the elective nature of implant surgery. Nerve damage can also occur when adjunctive high-risk procedures, such as bone harvesting and grafting, or nerve lateralization, are carried out to avoid implant-related nerve injury by increasing the clearance from the underlying vital structure (Yilmaz, et al., 2016).
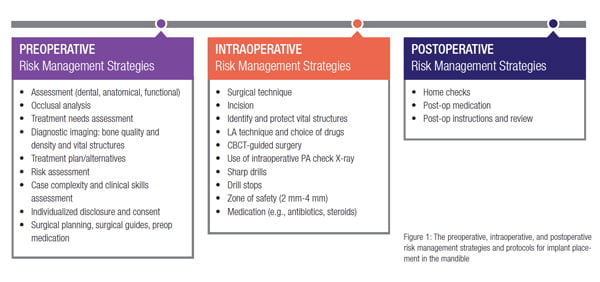
Systematic perioperative risk management strategies are needed in order to minimize collateral damage to the vital structures that may be located in close proximity of the implant sites when operating in the mandible. The risk management strategies presented in this article apply to all three distinct phases of implant treatment: preoperative, intraoperative, and postoperative (Figure 1).
Preoperative risk management strategies
In addition to a routine case assessment, a full periodontal examination should be carried out as well as adequate restorative and occlusal analysis, including the determination of the inter-arch clearance with articulated models. The aim is to document that restorative implant treatment is feasible, that tooth replacement is indicated for good functional reasons (a low, moderate, or strong indication, for example), that dental implants are the choice of treatment with respect to specific individual functional and anatomical conditions, and that the benefits outweigh the risks (such as TGI occurring, for example) of the case.
At this stage, individual treatment needs (TN) should be established as well as performing a complexity of treatment analyses, using an established index such as the ITI’s straightforward, advanced, and complex (SAC) classification, or the Cologne ABC risk assessment scores should be performed.
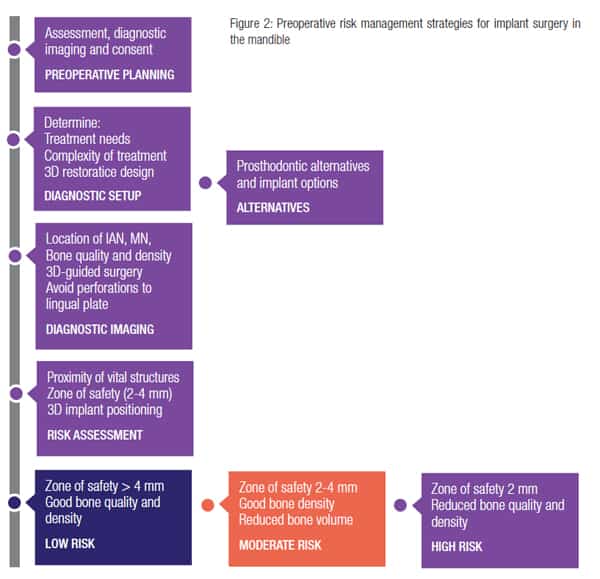
Case-specific factors affecting the long-term prognosis and the related risk:benefit analysis should be performed and discussed with patients in view of their autonomy, preferences, and concerns. The choice of the radiograph should allow the determination of the bone quality and density at each implant site, the location of the vital structures, and their potential proximity to the planned implant sites. Although the panoramic radiograph is a safe and reliable technique for assessing bone height and space when there is sufficient bone, a CBCT study has shown that it may be unsafe to use an arbitrary safety margin (of 2 mm-4 mm) and recommends that individual determination of a zone of safety should be made on a case-by-case basis (Parnia, et al., 2012) optimally by using a CBCT (Yilmaz, et al., 2016; 2017).
Given that there is evidence to suggest that the most common cause of TGI may be inadequate radiological assessment of the implant site, the use of small field-of-view (sFOV) CBCT may be justifiable for optimal preoperative surgical planning when placing implants in close proximity of vital structures particularly in presence of limited bone density or quantity. Carrying out a panoramic radiograph routinely at first, only to realize that a 3D cross-sectional image would be required for implant placement, would simply add to the radiological burden.
It should however be noted that CBCT scanning is an invasive technique and is still considered as a supplementary method of imaging in implant dentistry by most dental organizations. Nevertheless the radiological risk benefit analysis could be further improved if all of the objectives outlined in Figure 2 are also considered as part of the justification criteria when selecting the most optimum imaging technique in the mandible (Table 3).
During this stage, special considerations should be given to the strength of the need for tooth replacement using dental implants, the material risk of TGI occurring with reference to the patient’s individual anatomical condition and the proximity of the vital structures, the prognosis of the proposed treatment, and the likelihood of satisfactory treatment outcome if traditional prosthodontic alternatives were offered.
This will form the basis of the patient information and consent process in which the patient is entitled to receive a full disclosure of the material risks, including the likelihood of TGI with reference to their specific individual anatomical condition. In this respect, for the consent process to be valid (Montgomery, 2015), all material risks related to implant surgery in the mandible as outlined in Table 1 should be disclosed even if the risk has been quantified to be low (Figure 2).
Intraoperative risk management strategies
The aim of surgical planning is to facilitate the placement of dental implants with precision at the planned three-dimensional position with correct angulation, depth, and crestal location.
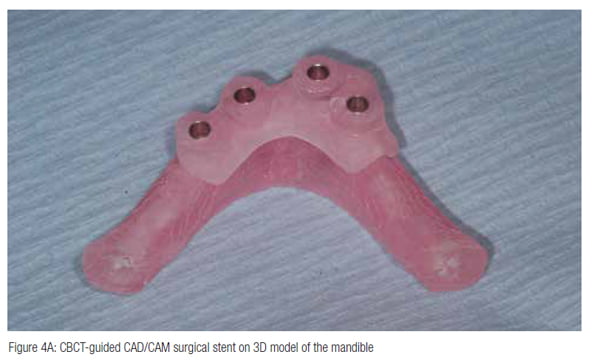
Freehand placement technique or use of a limited guidance laboratory fabricated surgical guide, although cheaper, could result in significantly more errors compared with CBCT-guided surgical stents (Block and Emery, 2016).
An intraoperative protocol of risk management strategies is outlined in Figure 3. Research evidence reported by Yilmaz, et al., (2016; 2017) and previous protocols used by Scher (2002) and Devine, Ucer, et al., 2014, have highlighted the importance of the following risk management strategies when placing implants in the mandible.
Surgical guides
CBCT-guided static stents have been shown to improve the surgical precision of implant placement if used with a coordinated drilling system (typically less than 2 mm apical and crestal deviation and less than 5º angulation error) and might reduce the risk of TGI (Block and Emery, 2016).
CBCT-based surgical planning (Figure 4) can also eliminate the chance of unforeseen anatomical anomalies or variations becoming apparent during the operation and forcing unplanned surgical deviations (Juodzbalys, et al., 2010; 2010). If preoperative planning can be completed with three-dimensional accuracy, the surgeon will not be forced to deviate from the planned surgery intraoperatively or violate the zone of safety that has been planned around the vital structure(s). Further studies, however, are needed in this area.
Zone of safety
In the UK survey, the majority of participants (76% of 134 responses) reported that they allowed a 2 mm-4 mm safety zone radiologically above the IAN when placing implants. Safety zones of more than 4 mm and less than 2 mm were allowed by 9% and 15% responders, respectively (Yilmaz, et al., 2016; 2017).
The authors recommend that a zone of safety should be determined individually (2 mm-4 mm) as a function of the available bone height above the nerve and minimum implant length that can be used predictably, using a CBCT scan (Figure 2).
Intraoperative X-rays
The authors recommend the use of intra-operative periodical check X-rays during implant placement surgery in order to reduce the risk of collateral damage to surrounding tissues.
Local anesthetic (LA)
Articaine was the most frequently cited LA used in the posterior mandible, followed by lignocaine. Nevertheless, a total of 73% of the responders reported that they did not use articaine in inferior dental blocks (IDB). Of those who encountered LA-related IAN injury (n = 62), pain on injection (17%), infection in proximity of the mental nerve (11%), use of articaine (8%), and multiple ID blocks (IDBs; 5%) were the main predictors of nerve damage related to LA during dental implant surgery.
It is clinically important to document any intraoperative findings such as pain on injection as these can help in postoperative diagnosis of the nature of the cause of NSD and how best to manage this complication (Yilmaz, et al., 2016; 2017).
In this study, 59% (n = 70) used infiltration anesthesia only for implant placement in the posterior mandible to avoid giving IDBs — presumably to reduce the risk of LA-related neurosensory disturbances as well as to prevent IAN injury during implant placement. Interestingly, LA infiltration technique was reported to be always effective by 71% (n = 84).
There is no agreement in the literature on using IDB or LA infiltration anesthesia during dental implant surgery in the posterior mandibular region. It has been argued that permanent IAN injury can be avoided if IDB is not administered to allow the patient to retain some sensory sensation during implant placement. Etoz, et al., 2011, showed that an LA infiltration was an effective method of anesthesia for implant surgery in the posterior mandible but recommended that the length of the implant should still be determined carefully to avoid possible damage to the IAN during implant placement.
 The authors, based on their audited long-term experience, routinely use and recommend an LA infiltration technique instead of IDBs when placing implants in the posterior mandible. However, the safety and effectiveness of this technique as a risk management strategy in reducing IAN or LN nerve damage is largely unknown but warrants further study.
The authors, based on their audited long-term experience, routinely use and recommend an LA infiltration technique instead of IDBs when placing implants in the posterior mandible. However, the safety and effectiveness of this technique as a risk management strategy in reducing IAN or LN nerve damage is largely unknown but warrants further study.
Flap design
 Identification and protection of a vital anatomical structure is a sound surgical practice, and clinicians should consider doing so when operating in close proximity of the IAN (Yilmaz, et al., 2016). Clinicians should be familiar with the surgical anatomy of the mandible. In moderate to severely atrophic mandibles, lingual and mental branches of the TG become more superficially located. Incision design should avoid damage to the mental and lingual nerves in particular.
Identification and protection of a vital anatomical structure is a sound surgical practice, and clinicians should consider doing so when operating in close proximity of the IAN (Yilmaz, et al., 2016). Clinicians should be familiar with the surgical anatomy of the mandible. In moderate to severely atrophic mandibles, lingual and mental branches of the TG become more superficially located. Incision design should avoid damage to the mental and lingual nerves in particular.
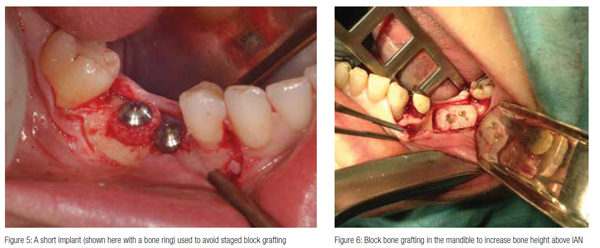
Short implants
The use of short implants (Figure 5) provides an alternative to bone grafting (Figure 6) in situations of limited bone volume and is becoming a viable alternative to longer implants that often require additional augmentation procedures — thus risking further NSD in the posterior mandible.
The current literature supports the use of short implants (6 mm-8 mm), which have been shown to have high survival rates after 5-10 years without marginal bone loss or complications and, therefore, can be employed for simplification of implant therapy in situations of reduced alveolar heights in the posterior jaw (Atieh, et al., 2012; Srinivasan, et al., 2012). Caution is however required when using short implants.
 The UK survey revealed that the most frequent standard length of implants used within the posterior mandible by (56%) was 10 mm. The most frequent, shortest implant length they would be happy to use in the molar region in the mandible were 6 mm and 8 mm, at 51 (39%) and 50 (38%), respectively (Yilmaz, et al., 2016; 2017). However, long-term randomized controlled trials are needed to show their long-term efficiency compared with longer implants.
The UK survey revealed that the most frequent standard length of implants used within the posterior mandible by (56%) was 10 mm. The most frequent, shortest implant length they would be happy to use in the molar region in the mandible were 6 mm and 8 mm, at 51 (39%) and 50 (38%), respectively (Yilmaz, et al., 2016; 2017). However, long-term randomized controlled trials are needed to show their long-term efficiency compared with longer implants.
Implant drills
 Manufacturers recommend their implant drills should ideally be one-use only or replaced frequently (after 10-15 cases). Most of the responding dentists questioned in a UK survey indicated that they replaced their surgical drills after 10 uses (35.9%). The second most popular choice indicated by the dentists (at 17.2%) was single-use only.
Manufacturers recommend their implant drills should ideally be one-use only or replaced frequently (after 10-15 cases). Most of the responding dentists questioned in a UK survey indicated that they replaced their surgical drills after 10 uses (35.9%). The second most popular choice indicated by the dentists (at 17.2%) was single-use only.
This was then followed by “after 20 uses” or “more than 20 uses,” at 16.4% and 12.5%, respectively. Nineteen responders (14.8%) did not have a policy for replacing implant drills, and a further 11 (8.6%) indicated that they had other policies regarding the replacement of implant drills.
The authors recommend that implant drills should be replaced according to the manufacturers’ recommendations so that they are sharp and efficient cutting instruments when placing implants in
the mandible.
Drill stops
Drill stops (Figure 7) appear to be used by only a small number of dentists in the United Kingdom.
Although the efficacy of using drill stops in this respect has not been demonstrated, they are seen as a good safety measure in preventing inadvertent extension of the osteotomy site or avoiding sudden sink of the drills when drilling in less dense bone (such as types D3 or D4). This should be investigated in future studies.
 Steroids
Steroids
In this study, steroids were used perioperatively always by 18%, while 18% used them when operating in borderline cases of limited bone volume. Antibiotics were also used prophylactically to reduce infection and the associated perineural edema. Efficacy of such medication is unknown, although Al-Bishri, et al., 2008, demonstrated a beneficial effect of a moderate perioperative dose of steroids on recovery of NSD that was mediated by recruitment of macrophages.
 The authors recommend the use of steroids (such as dexamethasone) when operating in close proximity of vital structures in the mandible unless there are systemic contraindications. Dexamethasone can be administered intravenously, subcutaneously, or intramuscularly. Oral formulations of steroids can also be considered pre- and postoperatively.
The authors recommend the use of steroids (such as dexamethasone) when operating in close proximity of vital structures in the mandible unless there are systemic contraindications. Dexamethasone can be administered intravenously, subcutaneously, or intramuscularly. Oral formulations of steroids can also be considered pre- and postoperatively.
Staging bilateral surgical procedure
It is recommended that bilateral implant placement in the mandible is staged in order to eliminate the possibility of bilateral nerve damage occurring. In the survey, 57% of the responders always staged bilateral implant placement although 19% did so only when bone volume was very limited.
Antibiotics
Routine use of preoperative antibiotics is recommended when placing implants in the mandible with or without adjunctive bone grafting procedures in order to reduce the risk of postoperative infection and possible nerve damage.
Lingual artery
When placing implants in the anterior mandible, perforation of the lingual plate should be avoided to prevent laceration injuries to the lingual artery branches (Figure 8). The precision of surgery can be improved significantly if a CBCT-guided surgical stent is used to ensure that implants are placed within the planned position, depth, and angulation (Figure 9).
Anatomical variations in nerve morphology
Morphological anatomical variations in the IAN or mental nerve are often noted intraoperatively and may force the surgeon to deviate from the intended course of action when placing implants. This could violate the planned safety zone and might result in inadvertent nerve damage.
In the UK survey, 86 of 122 responders (70%) noticed a large anterior loop, 16 (13%) encountered multiple canals, 13 (11%) noticed bifid mental nerve, and 21 (17%) noticed multiple mental foramina. CBCT planning would be highly indicated in order to identify anatomical variations in nerve morphology and should be considered (Yilmaz, et al., 2016; 2017).
Postoperative risk management strategies
Successful management of trigeminal nerve injury (TGI) depends on timely diagnosis of the mechanism of nerve injury. In case of IAN injury, the timing and duration of the injury is of particular relevance as the IAN is highly prone to irreversible ischemic changes if the nerve becomes compressed within its bony canal. This could occur due to infection, edema, hematoma formation, or pressure from bone debris (Renton, 2002; Yilmaz, et al., 2016; 2017).
Neurosensory disturbances once the LA has worn off should be investigated urgently after surgery in order to make a diagnosis and commence treatment without delay.
Home check
A home check within 6-12 hours after implant surgery is recommended to detect nerve injury as early as possible. The patient should be instructed both verbally and in writing to report back any neurosensory disturbance such as numbness, altered sensation, or intense pain that does not respond to usual postoperative analgesics once the LA has worn off. As the onset of nerve damage can be delayed, the patient should be followed closely, and any persistent infection should be treated adequately (Yilmaz, et al., 2016; 2017).
Diagnosis of trigeminal nerve injury
There are no clinical tests that can distinguish a bruised nerve from a sectioned nerve in the early postoperative phase. CBCT is not reliable in evaluating the outcome of IAN injury. Trigeminal sensory disturbances related to dental implants (Figure 10) must therefore be diagnosed subjectively according to patients’ complaints and symptoms.
It is also important to differentiate between TGI caused by LA administration or dental implant placement as this has a bearing on the management of the nerve damage (such as removal of the implant or not).
It is essential that clinicians make accurate intraoperative notes describing the key stages of the surgical procedure undertaken. This should include any unusual response such as a sudden give when drilling in close proximity of the nerve, or an “electric shock” that may be experienced by the patient when the LA is being administered. In order to effectively diagnose trigeminal nerve injury (TGI), a full clinical examination would be required.
The aim is to map out the area (and the dermatome) affected (the mucosa, skin, the lower lip, teeth, or the tongue, for example). The size of the area and the percentage of the dermatome affected will give an idea about the extent of the injury. Mechano-sensory tests such as light touch, sharp/blunt discrimination, pressure point pain, moving point allodynia, two-point discrimination, and thermal stimuli testing should be carried out and mapped.
Early referral
Clinicians who have not had specific training or experience in diagnosis and management of TGI should consider referring their patients to a specialist for prompt treatment of nerve injury as early as possible. The prognosis is affected by the mechanism as well as the timing and the duration of the injury. Prompt treatment could minimize the severity of the permanency of nerve damage.
The management of TGI depends on the mechanism of the injury and often on the severity of the patient’s symptoms. If the neuropathy affects most of the dermatome and is associated with severe neuropathic pain, medical management and early removal of the implant might be strongly indicated (Renton, 2013).
Conclusion
TGI is a devastating complication of dental implant surgery in the mandible. Given the fact that implant treatment is an elective surgery, this complication should be fully avoidable. The evidence suggests that TGI related to dental implants can be minimized with meticulous attention to accurate assessment, diagnosis, and treatment planning as well as carrying out the surgery with a high degree of precision.
In this article, the authors presented an outline of a protocol that comprises preoperative, intraoperative, and postoperative risk management strategies for dental implant surgical procedures in the mandible, based on a recent series of papers by Yilmaz, Ucer, Scher, Suzuki, and Renton (2016; 2017); Scher (2002), Devine, Ucer and Renton (2014), and Renton (2013).
References
- Al-Bishri A, Forsgren S, Al-Thobaiti Y, Sunzel B, Rosenquist J. Effect of betamethasone on the degree of macrophage recruitment and nerve growth factor receptor p75 immunoreaction during recovery of the sciatic nerve after injury: an experimental study in rats. Br J Oral Maxillofac Surg. 2008;46(6): 455-459.
- Atieh MA, Zadeh H, Stanford CM, Cooper LF. Survival of short dental implants for treatment of posterior partial edentulism: a systematic review. Int J Oral Maxillofac Implants. 2012;27(6):1323-1331.
- Block MS, Emery RW. Static or dynamic navigation for implant placement — choosing the method of guidance. J Oral Maxillofac Surg. 2016; 74(2):269-277.
- Devine M, Ucer C, Renton T. Incidence of Inferior Alveolar Nerve Injury with Mandibular Dental Implants. Paper presented at 43rd Annual Meeting & Exhibition of the American Association of Dental Research; March 19-24, 2014; Charlotte, NC.
- Etoz OA, Er N, Demirbas AE. Is supraperiosteal infiltration anesthesia safe enough to prevent inferior alveolar nerve during posterior mandibular implant surgery? Med Oral Patol Oral Cir Bucal. 2011;16(3):e386-e389.
- Juodzbalys G, Wang HL, Sabalys G. Anatomy of mandibular vital structures. Part I: mandibular canal and inferior alveolar neurovascular bundle in relation with dental implantology. J Oral Maxillofac Res. 2010; 1(1): e2.
- Juodzbalys G, Wang HL, Sabalys G. Anatomy of mandibular vital structures. Part II: Mandibular incisive canal, mental foramen and associated neurovascular bundles in relation with dental implantology. J Oral Maxillofac Res. 2010; 1(1):e3.
- Montgomery v Lanarkshire Health Board (2015) UKSC 11. On appeal from: (2013) CSIH 3; (2010) CSIH 104
- Parnia F, Moslehifard E, Hafezeqoran A, Mahboub F, Mojaver-Kahnamoui H. Characteristics of anatomical landmarks in the mandibular interforaminal region: a cone-beam computed tomography study. Med Oral Patol Oral Cir Bucal. 2012; 17(3):e420-e425.
- Renton T, Yilmaz Z. Profiling of patients presenting with posttraumatic neuropathy of the trigeminal nerve. J Orofac Pain. 2011;25(4): 333-344.
- Renton T (2013). Oral surgery: part 4. Minimising and managing nerve injuries and other complications. Br Dent J. 2011; 215(8):393-399.
- Renton T. Association of Dental Implantology (ADI) Guidelines. 2013. https://www.adi.org.uk/resources/guidelines_and_papers/guidance_on_inferior_alveolar_nerve_injury/index.html. Accessed January 2017.
- Scher EL. Risk management when operating in the posterior mandible. Implant Dent. 2002;11(1):67-72.
- Srinivasan M, Vazquez L, Rieder P, Moraguez O, Bernard JP, Belser UC. Efficacy and predictability of short dental implants (<8 mm): a critical appraisal of the recent literature. Int J Oral Maxillofac Implants. 2012;27(6):1429-1437.
- Tyndall DA, Price JB, Tetradis S, Ganz SD, Hildebolt C, Scarfe WC: American Academy of Oral and Maxillofacial Radiology. Position statement of the American Academy of Oral and Maxillofacial Radiology on selection criteria for the use of radiology in dental implantology with emphasis on cone beam computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113(6):817-826.
- Yilmaz Z, Ucer C, Scher E, Suzuki J, Renton T. A survey of the opinion and experience of uk dentists: Part 1: The incidence and cause of iatrogenic trigeminal nerve injuries related to dental implant surgery. Implant Dent. 2016;25(5):638-645.
- Yilmaz Z, Ucer C, Scher E, Suzuki J, Renton T. A survey of the opinion and experience of UK dentists: Part 2: Risk assessment strategies and the management of iatrogenic trigeminal nerve injuries related to dental implant surgery. Implant Dent. 2017;26(2):256-262.



