Educational aims and objectives
This article aims to describe the clinical indications of platelet-rich fibrin (PRF).
Expected outcomes
Implant Practice US subscribers can answer the CE questions by taking the quiz to earn 2 hours of CE from reading this article. Correctly answering the questions will demonstrate the reader can:
- Identify how PRF can be applied in implant dentistry, periodontology, oral surgery, and regenerative endodontics.
- Read some evidence-based literature on the use of PRF in implant dentistry.
- Realize where and how to use PRF.
- Discuss sinus floor elevation using PRF as sole or combination graft biomaterial.
- Discuss alveolar ridge preservation (socket augmentation) for early or late implant placement.
- Discuss immediate post-extraction implant placement and jump-gap augmentation – peri-implant healing.
- Realize various aspects of augmentation of dehiscence and fenestration defects.
- Identify treatment of peri-implant osseous defects.
- Realize various aspects regarding guided bone regeneration and periodontal surgery.
Drs. Johan Hartshorne and Howard Gluckman discuss the clinical indications of PRF in implant dentistry, periodontology, oral surgery, and regenerative endodontics
Platelet-rich fibrin (PRF) is a patient blood-derived living biomaterial with applications in a wide range of fields, including implant dentistry, periodontics, oral surgery, and regenerative endodontics. Improving one’s understanding of the clinical indications of this material will facilitate the ability to enhance the therapeutic applications of PRF in these fields, and this is the purpose of this article.
Introduction
Key treatment objectives in dental implantology, periodontology, and oral surgery are the prospect of having new therapies, biomaterials, and bioactive surgical additives available that will improve success and predictability of patient outcomes in soft and bone tissue healing and regeneration.
PRF, a patient blood-derived and autogenous living biomaterial, is increasingly being investigated and used worldwide by clinicians as an adjunctive autologous biomaterial to promote bone and soft tissue healing and regeneration. The gold standard for in vivo tissue healing and regeneration requires the mutual interaction between a scaffold (fibrin matrix), platelets, growth factors, leukocytes, and stem cells (Kawase, 2015). These key elements are all active components of PRF and, when combined and prepared properly, are involved in the key processes of tissue healing and regeneration, including cell proliferation and differentiation, extracellular matrix synthesis, chemotaxis and angiogenesis (neovascularization) (Dohan, et al., 2012; 2014). An improved understanding of the development, biological and physiological properties, and characteristics of PRF in tissue healing and regeneration over the past 2 decades has led to more successful therapeutic applications, especially in the fields of implant dentistry, periodontology, and oral surgery.
Methodology, search strategy, and inclusion criteria
An electronic MEDLINE® (PubMed®) and Google Scholar search was performed for all articles on platelet-rich fibrin (PRF) and platelet concentrates up to May 2016. The search was complemented by an additional hand search of selected journals in oral implantology, oral surgery and periodontal, as well as gray literature. The reference lists and bibliographies of all included publications were also screened for relevant studies. The search was limited to the English language. Randomized controlled trials (RCTs), controlled clinical trials (CCTs), case reports, case series, prospective, retrospective and in-vitro/in vivo studies were included in the narrative review. Animal studies were excluded from this review.
Where and how can I use PRF?
A general rule of guidance is to use PRF in surgical situations where protection and stimulation of healing and regeneration is critical and where the prognosis for tissue repair is poor or potentially compromised in the absence of a tissue regeneration scaffold and addition of growth factors.
Implant dentistry
Most of the PRF clinical research in implantology is currently focused in the fields of improving clinical outcomes with sinus floor elevations using PRF as sole grafting material, simultaneous with implant placement (Ali, et al., 2016; Simonpieri, et al., 2011) or SFE using a combination of PRF and bone allograft (FDBA) prior to implant placement (Choukroun, et al., 2006; Tatullo, et al., 2012).
Other focus areas of clinical research are use of PRF in alveolar ridge preservation (socket augmentation) (Hauser, et al., 2013), peri-implant tissue healing (Boora, et al., 2015), and improving implant stability (Öncu and Alaaddinoõglu 2015) (Table 1).
In vitro studies have shown PRF-induced gene expression of the early and late markers of osteogenesis stimulates bone and soft tissue healing (Clipet, et al., 2012). This finding suggests that PRF is indicated in numerous clinical applications, such as socket augmentation, jump gap filling during immediate extraction and implant placement, and stimulation of bone and soft tissue healing during bone augmentations or during sinus elevation.
Sinus floor elevation using PRF as sole or combination graft biomaterial
A systematic review showed that PRF used as a sole filling material in SFE with simultaneous implant placement is a simple technique with promising results (Ali, et al., 2016; Kanayama, et al., 2016). Various clinical case reports describe the lateral approach for sinus floor elevation using only PRF as the grafting material (Mazor, et al., 2009; Simonpieri, et al., 2011; Tajim, et al., 2013) (Figures 1A-1C).
The PRF membrane is recommended as an inexpensive and easily handled substitution biomaterial during sinus elevation to reduce the healing time before loading. It is a cheap and easily handled material with healing properties. Its fibrin matrix properties and ability of slowly releasing growth factors make it an ideal replacement biomaterial to replace xenogenic and expensive collagen membranes in some situations (Mazor, et al., 2009).
The review also suggests that PRF combined with a bone allograft or other bone substitutes accelerates graft maturation and decreases the healing period before implant placement. (Figures 2A-2E) The latter finding has also been confirmed by other clinical trials and case studies (Choukroun, et al., 2006; Tatullo, et al., 2012; Zhang, et al., 2012; Bölükbas, et al., 2013).
Various case studies have demonstrated that PRF membranes can be used successfully as a protective barrier to cover the sinus membrane during grafting procedures (Diss, et al., 2008; Toffler, et al., 2010; Kanayama, et al., 2014) (Figure 3). PRF membranes also represent an easy and successful method to cover sinus membrane or osteotomy window to protect the Schneiderian membrane, to facilitate wound closure, and to enhance healing (Ghetiu, et al., 2015). Cases have also been reported showing that A-PRF membrane can be used as a healing barrier when perforations or tears of the Schneiderian membrane occur (Diss, et al., 2008; Toffler, et al., 2010).
Alveolar ridge preservation (socket augmentation) for early or late implant placement
Use of PRF membranes to fill the socket after tooth extraction has shown to improve alveolar bone healing and preservation of the alveolar crest width. PRF plugs or membranes can also be used to fill extraction sockets, even when associated with compromised extraction sockets (Peck, et al., 2011), severe cystic destructions, or after cyst enucleations (Choukroun, et al., 2006; Magremanne, et al., 2009) to allow early bone and gingival regeneration required for implant placement. Clinical and histological findings suggest that filling a fresh extraction socket with PRF provides a viable therapeutic alternative for implant site preparation (Zhao, et al., 2011).
Alternatively, PRF can also be mixed with a bone substitute to fill the socket and used as a protective cover over the grafted socket (Figures 4A-4G). This is particularly important when gingival wound closure is impossible or difficult with the sutures (Del Corso, et al., 2012).
The purpose of the PRF membrane is not only to stimulate gingival healing, but also to protect the bone graft from the oral environment and to maintain it within the extraction socket, like a biological barrier. It is suggested that this technique negates the need for using more complex flaps and GBR protocols to close and augment extraction sockets.
Immediate post-extraction implant place-ment and jump-gap augmentation — peri-implant healing
PRF can be considered as a healing biomaterial with potential beneficial effect on peri-implant tissue and can be used as a therapeutic adjuvant with immediate implant placement in the clinical scenario of one-stage, single-tooth implant placement procedure in maxillary anterior region (Del Corso, et al., 2012).
With immediate implant placement, the peri-implant jump gap can be augmented with PRF clot (A-PRF or L-PRF) or solution (i-PRF) mixed with a bone substitute (Rao, et al., 2013) (Figures 5A and 5B).
In the latter case study, it is suggested that the augmented jump gap is covered with cross-linked collagen membrane, overlaid by a double layer of PRF and the flap closed by sutures. Studies have demonstrated that the use of leukocyte-platelet rich fibrin (L-PRF or A-PRF) membranes for the stimulation of bone and gingival healing around the implant is particularly significant (Öncü and Erbeyo 2015) (Figures 6A-6C). The elastic consistency of the PRF membrane allows the clinician to punch a hole in the membrane to facilitate draping the membrane over the healing abutment (Figures 7A and 7B).
Augmentation of dehiscence and fene-stration defects
Case reports indicate that PRF membrane cut in pieces or i-PRF combined with bone substitutes may offer an easy and simple method of handling and delivery of a fibrin scaffold, growth factors, and cells during the augmentation of dehiscence and fenestration defects, and at the same time reduce soft tissue and bone healing time (Simonpieri, et al., 2009; 2009; Toeroek and Dohan 2013).
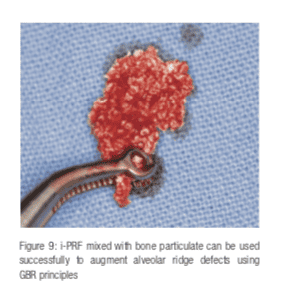
PRF has also been successfully used to treat fenestration defects around implants (Vijayalakshmi 2012). PRF membrane cut in pieces (Figure 8), or platelet liquid (I-PRF) (Figure 9) can be mixed with bone graft material to cover the defect. The graft is covered with a resorbable collagen membrane to maintain form and shape and to confer graft stability and space maintenance on the bone particles. PRF membrane is then placed on top of the collagen membrane to prevent tissue dehiscence and aid in soft tissue healing.
 The application of PRF to GBR procedures offers several advantages including promoting wound healing, bone growth, and maturation, graft stabilization, wound sealing, and hemostasis, and improving the handling properties of graft materials.
The application of PRF to GBR procedures offers several advantages including promoting wound healing, bone growth, and maturation, graft stabilization, wound sealing, and hemostasis, and improving the handling properties of graft materials.
Treatment of peri-implant osseous defects
One clinical study showed that treatment of peri-implant defects with PRF was clinically more effective than with conventional flap surgery alone, irrespective of the type of defect (Hamzacebi, et al., 2015).
In another study, a successful treatment outcome was also reported after debridement and detoxification with a Cr,CR:YCGG later, followed by filling the defect with a synthetic hydroxyapatite embedded in native blood and covered with a PRF membrane to prevent soft tissue infiltration into the grafted area. The authors concluded that the use of PRF added to the maintenance of the graft homeostasis due to release of growth factors, thus contributing to the successful outcome of treatment (Shilbli, et al., 2013).
Augmentation of alveolar ridges (horizontal and vertical) and buccal bone defects (GBR)
Several cases have been reported of successful augmentation of alveolar ridges where there is a buccal bone defect (GBR) using PRF combined with a bone substitute (Inchingolo, et al., 2010; Krasny, et al., 2011; Kim, et al., 2013; Joseph, et al., 2014) and in cases of the severely resorbed posterior mandible.
Del Corso and Dohan (2013) suggest that three layers of L-PRF membranes used alone were adequate to use as competitive interposition barrier to protect and stimulate the bone compartment, and as healing membranes to stimulate the periosteum and gingival healing and remodeling. Periosteal incisions were done on the flaps to promote their tension-free closure.
The concept of using PRF alone as a GBR barrier still raises many questions as well as limitations and needs to be investigated with robust RCTs to determine appropriate indications and relevant combinations (Toeroek and Dohan 2013). PRF liquid (i-PRF) can be injected, or PRF membrane placed above the GBR, or GTR membrane to act as an interposition barrier to protect and stimulate the bone compartment and as a healing membrane in order to improve the soft tissue healing and remodeling, and thus avoid soft tissue dehiscence.
Guided bone regeneration
Place harvested autogenous bone adjacent to the implant or defect that requires grafting. The next layer is made up of a mixture of bone grafting material with i-PRF or PRF membrane or clot cut in small pieces.
The objective of this mixture is to help the rapid vascularization of the bone grafting material through the PRF fibrin matrix making the bridge between bone particles and allowing a quick new bone growth, while the xenograft material serves as space maintainer for the regenerative volume and supports the nucleation and accumulation of newly formed bone matrix.
The bone/PRF mixture in the augmented site is covered with a cross-linked collagen membrane to maintain the bone compartment and to prevent ingrowth of soft tissue. The collagen membrane is overlaid with a double layer of PRF membranes (Figures 10A-10C). These membranes are used as a competitive interposition barrier to protect and stimulate the bone compartment, and as healing membranes to stimulate the periosteum and gingival healing and remodeling. Periosteal releasing incisions are done on the flaps to promote their tension-free closure.
Increasing implant stability and osseo-integration
Case studies suggest that PRF application into the osteotomy site (Figures 11A-11C) increases implant stability during the early healing period, as evidenced by higher ISQ values. Simple application of this material also seems to provide faster osseointegration (Öncu and Alaaddinoõglu 2015).
Periodontal surgery
Most of the clinical research in periodontology is currently focused in the fields of improving clinical outcomes with treatment of intrabony periodontal pockets, furcation defects, gingival recession defects, and healing of connective tissue graft sites in the palate (Table 2).
Intrabony and furcation periodontal defects
The regenerative and wound healing effects have been very promising with PRF treating intrabony defects (see also Table 3).
The clinical evidence is supported by several case studies reporting on the successful application of PRF to regenerate bone and gingival tissues around teeth presenting with intrabony defects and furcation lesions.
Another study has also reported on using PRF successfully as a regenerative material in cases of aggressive periodontitis (Desarda, et al., 2013).
Surgical periodontal therapy accompanying the placement of PRF in angular defects of aggressive periodontitis patients showed decreased probing pocket depth, increased attachment level, and radiographic bone fill when baseline and 9-month follow-up data was compared. Surgical reconstructive therapy with placement of PRF in angular defects can is suggested as an effective approach to enhance periodontal regeneration.
Platelet gel acts as a stabilized blood clot and therefore is recommended as a perfect filling material for natural tissue regeneration and healing. Clinically, the general concept of “natural tissue regeneration” (NTR) and natural bone regeneration (NBR) (Del Corso, et al., 2012) requires to fill the periodontal intrabony defect with L-PRF, most times in association with a bone substitute used as a solid space maintainer, and then to cover the filled intrabony defect with L-PRF membranes, used for the protection of the grafted area and as a healing booster for the soft tissues above the defects. The objective of this cover is not only to protect the blood clot and/or the filling material, like in the GTR concept, but also to promote the induction of a strong and thick periosteum and gingiva.
The boosted periosteum functions as a true barrier between the soft tissue and bone compartments, and constitutes probably the best protection and regenerative barrier for the intrabony defects. The NTR protocol is very simple and gives excellent results in most clinical situations, with no contraindication or risk of negative effects. However, in order to get the best results, the choice and the quantity of the adequate bone substitute has yet to be determined in various clinical configurations.
Theoretically, PRF can be used as a sole grafting material or in combination with bone substitutes can be used as a filling material in intrabony defects, following GTR principles. PRF membrane is a solid material with the advantage that it is easy to handle and to position in bony defects. PRF membranes can also be used as a protection membrane after the filling of the intrabony defect (Figures 12A and 12B). In comparison to GTR membranes, PRF will undergo a quicker remodeling in situ than a resorbable collagen membrane, but will promote a strong induction on the periosteum and gingival tissue due to the slow release of growth factors and other matrix proteins (Dohan 2009; Del Corso, et al., 2009; Dohan, et al., 2009).
GTR membranes are cell-proof barriers, whereas a PRF membrane is a highly stimulating matrix, attracting cell migration and preferential differentiation allowing new blood vessel formation within the matrix, and also reinforcing the natural periosteal barrier. The hard and soft tissues migrate and interact within the PRF matrix. The PRF matrix becomes the interface between the tissues and therefore avoids the migration of the soft tissues deeper within the grafted defect or augmented site. This biological characteristic is referred to as a competitive barrier.
These bioactive interactions are very important during tissue regeneration since the periosteum covers the internal part of the gingival flap and is a key actor of bone healing and gingival maturation. While GTR membranes block the periosteum healing potential and bone/gingival interactions, PRF membranes stimulate the periosteum’s regenerative properties. However, even if PRF membranes do not block the migration of the cells, no invagination of the soft tissues within the bone area was observed when PRF membranes covered a filled intrabony defect (Simonpieri, et al., 2011).
Recession defects and guided tissue regeneration (GTR)
The clinical evidence is less promising with the treatment of recession defects (Moraschini, et al., 2015; Keceliet, et al., 2015; Rajaram, et al., 2015) (Table 2). Within the limitations of available clinical trials, the clinical evidence indicates that PRF does not improve root coverage or increases the width of keratinized mucosa in Miller’s Class I and II gingival recessions, compared to other treatment modalities.
However, cases have been reported where PRF was successfully used for treating localized (Eren and Atilla 2014; Aroca, et al., 2009; Aleksic, et al., 2010; Jankovic, et al., 2010; Jankovic, et al., 2012) and multiple (Agarwal, et al., 2013) gingival recessions. Another case study reported that the use of PRF membrane along with the VISTA technique allows clinicians to successfully treat multiple recession defects with optimal esthetic results and excellent soft tissue biotype (Gupta, et al., 2015).
Palatal bandage
PRF membranes can also be used as a palatal wound bandage or protection membranes after harvesting connective tissue grafts in the palate (Femminella, et al., 2015; Kulkarni, et al., 2014; Jain, et al., 2012).
Case studies show that PRF membrane used as a palatal bandage is an efficacious approach to protect the raw wound area of a palatal donor site and significantly accelerates palatal wound healing and reduces patient discomfort and healing time (Aravindaksha, et al., 2014; Femminella, et al., 2016).
Interdental papilla augmentation
A case study reported that PRF combined with bone graft for regeneration of interdental bone may contribute towards improved clinical success with augmentation of lost dental papilla (Arunachalam, et al., 2012). The reconstructed papilla in the new position was stable when reviewed at 3 and 6 months postoperatively.
Oral surgery
Most of the evidence-based research in the field of oral surgery is primarily focused on enhancing bone healing (Singh, et al., 2012; Gurbuzer, et al., 2010; Rao, et al., 2013) and reducing postoperative complications following third molar extractions. Research is also emerging on promoting healing of apico-marginal defects in root end surgery (Table 3). Numerous case and case series studies have been reported that support the use of PRF in various clinical applications in the field of oral surgery.
Post-extraction socket augmentation and healing
The healing and remodeling of an extraction socket is highly dependent on the initial stabilization of the blood clot and the quick gingival wound closure. This can be achieved by placing fibrin plug in the socket (with or without a bone substitute) and closing with a fibrin membrane. The use of PRF as a post extraction sockets filling biomaterial is recommended as a useful procedure in order to reduce the early adverse effects of the inflammation, such as postoperative pain (Eshghpour, et al., 2014; Uyanik, et al., 2015) and to promote the soft tissue healing and bone regeneration process (Manernzi, et al., 2015).
Clinical situations where post-extraction socket augmentation with PRF is specifically indicated are for early or delayed implant placement and immediate post-extraction implant placement (Simon, et al., 2009; Simon, et al., 2011; Triveni, et al., 2012; Rao, et al., 2013; Basarli, et al., 2015).
Reduce post-extraction complications in medically compromised cases
PRF can be used to minimize post-extraction complications such as osteitis, dry or infected sockets resulting from delayed or potentially compromised healing or bleeding situations in systemic conditions such as such as with diabetics, patients receiving oral bisphosphonate medication presenting a risk of osteonecrosis of the jaws, or patients receiving anticoagulants (Sammartino, et al., 2011).
Delayed or compromised healing of extraction sockets is mostly related to an unstable blood clot within the socket. In such case a fibrin clot is simply placed in the socket, covered with a collagen plug or membrane and sutured. Placing PRF in a socket could amplify the natural coagulation process and enhance socket healing.
Prevention of periodontal complications in third molar surgery
Complex third molar extractions frequently result in critical size bone defects and compromised healing impacting negatively on the outcome of periodontal tissues distal of the second molar. When bone defects after extraction are critical-sized (and often associated with cystic lesions), using PRF as a filling material or mixing PRF with a bone substitute in order to use a significant volume of solid biomaterial for filling is considered as reliable option.
These treatments are not, however, simple dental extractions, and are often at the border of guided bone regeneration (GBR) or bone grafting. The use of a PRF as a filling material significantly promotes soft tissue healing and also faster regeneration of bone in these sites and neighboring periodontal tissues (Ruga, et al., 2011; Yelamai and Saikrishna 2014).
Closing oroantral fistulas
Oroantral communications can complicate dental surgery, particularly during extraction of a posterior maxillary root. PRF clots can be used successfully for atraumatic or minimal intervention closure of oroantral communications, thus eliminating the need to raise mucoperiosteal buccal sliding flaps (Gülen, et al., 2015). The technique for closure of an oroantral fistula using platelet-rich fibrin is described by Argawal and co-workers (2016).
Apical/root end surgery
PRF clot (gel) serves as an ideal scaffold in root-end surgical procedures to enhance soft tissue healing and bone regeneration (Kuz’minykh 2009; Singh, et al., 2013; Shivashankar, et al., 2015; Nagaveni, et al., 2015).
Other researchers (Dhiman, et al., 2015) report that PRF may not necessarily improve the outcome of treatment.
The combination of PRF membrane as a matrix and MTA can prove to be an effective alternative for creating artificial root-end barriers and to induce faster periapical healing with large periapical lesions (Kathuria, et al., 2011). It is suggested that the combination of PRF and β-TCP for bone augmentation in treatment of periapical defects is also a more effective at increasing healing time compared to using bone substitute material alone (Jayalakshmi, et al., 2012). PRF combined with an alloplastic bone substitute has been successfully used for the management of combined endodontic-periodontal lesions (Goyal 2014).
Other oral surgical applications
The use of PRF has also been reported in other therapies including: alveolar cleft grafting (Findik and Byakul 2013).
Bisphosphonate-related oral necrosis of the jaw
Recent case reports suggested that PRF might stimulate gingival healing and act as a barrier membrane between alveolar bone and the oral cavity, therefore offering a simple, though effective treatment for the closure of bone exposure in bisphosphonate-related oral necrosis of the jaw (BRONJ) (Soydan and Uckan 2014; Kim, et al., 2014; Lundquist, et al., 2008; Nørholt and Hartley 2016).
Compromised wound healing situations (diabetics)
PRF has also been used as an adjuvant in the management of problematic chronic wounds (Lundquist, et al., 2008). The authors have shown that growth factors in PRF are protected from proteolytic degradation. This may be advantageous in the treatment of chronic wounds characterized by high proteinase activity.
Regenerative endodontic therapy
Regenerative endodontic procedures are widely being added to the current armamentarium of pulp therapy procedures (Huang, et al., 2010; Hotwani and Sharma 2014; Khetarpal, et al., 2013; Li, et al., 2013).
These biologically based procedures are designed to restore function of a damaged and non-functioning pulp by stimulation of existing dental pulp stem and progenitor cells present in the root canal under conditions that are favorable to their differentiation.
Management of open apex
Recent case reports have shown that the combined use of platelet-rich fibrin (PRF), and mineral trioxide aggregate (MTA) as root filling material is beneficial for the endodontic management of an open apex (Sundar, et al., 2015; Woo, et al., 2016). It is hypothesized that the combination of MTA and PRF may have a synergistic effect on the stimulation of odontoblastic differentiation of stem cells.
Revascularization and revitalization
Revascularization is the most studied and successful approach of regenerative endodontics (Sundar, et al., 2015). Revitalization of necrotic infected immature tooth is possible under conditions of total canal disinfection combined with the additive effect of PRF (Shivashankar, et al., 2012; Nagaveni, et al., 2015). PRF is proposed as an ideal biomaterial for pulp-dentin complex regeneration because it is a potentially valid scaffold material containing leukocyte and growth factors to facilitate tissue healing and regeneration in immature necrotic teeth in children (Woo, et al., 2016).
Repair and regenerative potential of PRF and enhanced cellular metabolism with laser bio stimulation, in combination with the sealing ability of MTA enhances the clinical success outcomes in pulpotomy and apexification procedures (Sundar, et al., 2015). Revitalization, revascularization, and regenerative pulp therapies still need to be validated with robust clinical trials.
Conclusion
PRF is increasingly being investigated and used by clinicians worldwide as an adjunctive autologous biomaterial to promote bone and soft tissue healing and regeneration. PRF technology has grabbed the attention of clinicians for several reasons: PRF is derived from the patients’ own blood, is readily available, can be produced immediately at the chairside, and is easy to prepare and is easy to use.
Furthermore, PRF technology is widely applicable in dentistry, while being financially realistic for the patient and the clinician, and with virtually no risk of a rejection reaction (foreign body response).
Clinicians are using PRF extensively and successfully in various clinical applications in dental implantology, periodontology, maxillofacial and oral surgery, and lately in regenerative endodontics to promote wound healing and tissue regeneration. The use of PRF in revitalization, revascularization, and regenerative pulp therapies are currently attracting a lot of attention, and several case studies in the field of regenerative endodontics are being reported. These applications, however, still need to be validated with robust clinical trials.
One of the clinical limitations to note is the heterogeneity in the quality of platelets and blood components due to use of different PRF preparation protocols in the various studies reviewed. Irrespective of the protocol used, all studies have all reported successful outcomes with regards to soft and bone tissue healing and regeneration. It should also be noted that at this stage in time there is not a single RCT or CCT to compare the effectiveness of A-PRF or L-PRF protocols. Furthermore, in vitro studies that claim superiority or inferiority of a specific PRF preparation have yet to be validated by independent clinical trials.
The future of PRF and its applications in clinical dentistry, especially in the field of soft tissue and bone regeneration, has enormous implications, but developing and strengthening its role in dentistry is dependent on its coherence and scientific clarity. The clinical effectiveness of different PRF preparation protocols in various clinical settings remains to be validated with a greater number of independent and robust RCTs, preferably with a split-mouth design, and larger sample sizes.
Independent, coherent, and scientific validation of PRF is needed to enhance the potential of this technology, thereby extending its therapeutic applications with improved successful and predictable outcomes for the benefit of the patient. PRF technology is in its infancy and will in future have a big impact in dentistry.
The benefits derived from the using PRF in various clinical applications for promoting wound healing and tissue regeneration, its antibacterial and anti-hemorrhagic effects, the low risks with its use, and the availability of easy and low-cost preparation methods should encourage more clinicians to adopt this technology in their practice for the benefit their patients[/vc_column_text][vc_column_text]Missed the first parts of this interesting and informative series on promoting tissue healing and regeneration with platelet-rich fibrin? Check out part 1 here.
References
- Agarwal K, Chandra C, Agarwal K, Kumar N. Lateral sliding bridge flap technique along with platelet rich fibrin and guided tissue regeneration for root coverage. J Indian Soc Periodontol. 2013;17(6):801-805.
- Agarwal B, Pandey S, Roychoudhury A. New technique for closure of an oroantral fistula using platelet-rich fibrin. Br J Oral Maxillofacial Surg. 2016;54():e31–e32
- Aleksić Z, Janković S, Dimitrijević B, et al. The use of platelet rich fibrin membrane in gingival recession treatment [in Serbian]. Srp Arh Celok Lek. 2010;138(1-2):11-18.
- Ali S, Bakry SA, Abd-Elhakam H. Platelet rich fibrin in maxillary sinus augmentation: a systematic review. J Oral Implantol. 2015;41(6):746-753.
- Aravindaksha SP, Batra P, Sood V, Kumar A, Gupta GT. Use of platelet-rich fibrin (PRF) membrane as a palatal bandage. Clin Adv Periodontics. 2013;4:1-6.
- Aroca S, Keglevich T, Barbieri B, Gera I, Etienne D. Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet-rich fibrin membrane for the treatment of adjacent multiple gingival recessions: a 6-month study. J Periodontol. 2009;80(2):244-252.
- Arunachalam LT, Merugu S, Sudhakar U. A novel surgical procedure for papilla reconstruction using platelet rich fibrin. Contemp Clin Dent. 2012;3(4):467-470.
- Bajaj P, Pradeep AR, Agarwal E, et al. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of mandibular degree II furcation defects: a randomized controlled clinical trial. J Periodont Res. 2013;48(5):573-581.
- Barone A, Ricci M, Romanos GE, et al. Buccal bone deficiency in fresh extraction sockets: a prospective single cohort study. Clin Oral Implants Res. 2015;26(7):823-830.
- Bansal C, Bharti V. Evaluation of efficacy of autologous platelet-rich fibrin with demineralized freeze dried bone allograft in the treatment of periodontal intrabony defects. J Indian Soc Periodontol. 2013;7(3):361-366.
- Baslarli O, Tumer C, Ugur O, Vatankulu B. Evaluation of osteoblastic activity in extraction sockets treated with platelet- rich fibrin. Med Oral Patol Oral Cir Bucal. 2015;20(1) e111-e116
- Bolukbasi N, Ersanli S, Keklikoglu N, Basegmez C, Ozdemir T. Sinus augmentation with platelet-rich fibrin in combination with bovine bone graft versus bovine bone graft in combination with collagen membrane. J Oral Implantol. 2015;41(5):586-595.
- Boora P, Rathee M, Bhoria M. Effect of Platelet Rich Fibrin (PRF) on Peri-implant Soft Tissue and Crestal Bone in One-Stage Implant Placement: A Randomized Controlled Trial. J Clin Diagn Res. 2015;9(4):ZC18-ZC21.
- Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):299-303.
- Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:299-303
- Clipet F, Tricot S, Alno N, et al. In vitro effects of Choukroun’s platelet-rich fibrin conditioned medium on 3 different cell lines implicated in dental implantology. Implant Dent. 2012;21:51-56.
- Del Corso M, Sammartino G, Dohan EDM. Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet-rich fibrin membrane for the treatment of adjacent multiple gingival recessions: a 6-month study. J Periodontol. 2009;80(11):1694-1697.
- Del Corso M, Vervelle A, Simonpieri A, et al. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 1: Periodontal and dentoalveolar surgery. Curr Pharm Biotechnol. 2012;13(7):1207-1230.
- Del Corso M, Mazor Z, Rutkowski JL, Dohan Ehrenfest DM. The use of leukocyte- and platelet-rich fibrin during immediate post extractive implantation and loading for the aesthetic replacement of a fractured maxillary central incisor. J Oral Implantol. 2012;38(2):181-187.
- Del Corso M, Dohan EDM. Immediate implantation and peri-implant Natural Bone Regeneration (NBR) in the severely resorbed posterior mandible using Leukocyte- and Platelet-Rich Fibrin (L-PRF): a 4-year follow-up. POSEIDO. 2013;1(2):109-116.
- Desarda HM, Gurav AN, Gaikwad SP, Inamdar SP. Platelet rich fibrin: a new hope for regeneration in aggressive periodontitis patients: report of two cases. Indian J Dent Res. 2013;24(5):627-630.
- Dhiman M, Kuman S, Duhan J, Sangwan P, Tewari S (2015). Effect of Platelet-rich Fibrin on Healing of Apicomarginal Defects: A Randomized Controlled Trial. J Endod. 41(7):985-991
- Diss A, Dohan DM, Mouhyi J, Mahler P. Osteotome sinus floor elevation using Choukroun’s platelet-rich fibrin as grafting material: a 1-year prospective pilot study with microthreaded implants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(5):572-579.
- Dohan EDM, de Peppo GM, Doglioli P, Sammartino G. Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors. 2009;27(1):63-69.
- Dohan EDM. How to optimize the preparation of leukocyte- and platelet-rich fibrin (L-PRF, Choukroun’s technique) clots and membranes: introducing the PRF Box. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(3):275-278; author reply 8-80.
- Dohan EDM, Bielecki T, Mishra A, et al. In search of a consensus terminology in the field of platelet concentrates for surgical use: platelet-rich plasma (PRP), platelet-rich fibrin (PRF), fibrin gel polymerization and leukocytes. Curr Pharm Biotechnol. 2012;13(7):1131-1137.
- Dohan Ehrenfest DM, Andia I, Zumstein MA, et al. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014;4(1):3-9.
- Eren G, Atilla G. Platelet-rich fibrin in the treatment of localized gingival recessions: a split-mouth randomized clinical trial. Clin Oral Invest. 2014;18(8):1941-1948
- Eshghpour M, Dastmalchi P, Nekooei AH, Nejat AH. Effect of platelet-rich fibrin on frequency of alveolar osteitis following mandibular third molar surgery: a double-blinded randomized clinical trial. J Oral Maxillofac Surg. 2014;72(8):1463-1467.
- Femminella B, Iaconi MC, Di Tullio M, et al. Clinical Comparison of Platelet Rich Fibrin and a Gelatin Sponge in the Management of Palatal Wounds Following Epithelialized Free Gingival Graft Harvest: A Randomized Clinical Trial. J Periodontol. 2015;87(2):103-113.
- Findik Y, Baykul T. Secondary closure of alveolar clefts with mandibular symphyseal bone grafts and with platelet-rich fibrin under local anesthesia: three case reports. J Contemp Dent Pract. 2013;4(4):751-753.
- Gamal AY, Abdel Ghaffar KA, Alghezwy OA. Crevicular Fluid Growth Factors Release Profile Following the Use of Platelets Rich Fibrin (PRF) and Plasma Rich Growth Factors (PRGF) in Treating Periodontal Intrabony Defects (Randomized Clinical Trial). J Periodontol. 2016;87(6):654-662.
- Ghetiu A, Sirbu D, Topalo V, et al. Tissue engineering with Platelet-Rich-Fibrin in Oral region. Clin Oral Impl Res. 2015;26(suppl 12):205.
- Goyal L. Clinical effectiveness of combining platelet rich fibrin with alloplastic bone substitute for the management of combined endodontic periodontal lesion. Restor Dent Endod. 2014;39(1):51-55.
- Gülen U,Entürk MF, Mehdiyev. Flap-free treatment of an oro-antral communication with platelet-rich fibrin. Br J Oral Maxillofac Surg. 2016;54(6):702-703.
- Gupta SJ, Jhingran R, Gupta V, et al. Efficacy of platelet-rich fibrin vs. enamel matrix derivative in the treatment of periodontal intrabony defects: a clinical and cone beam computed tomography study. J Int Acad Periodontol. 2014;16(3):86-96.
- Gupta G, Puri K, Bansal M, Khatri M, Kumar A. Platelet-rich fibrin-reinforced vestibular incision subperiosteal tunnel access (VISTA) technique for recession coverage. Clin Adv Periodontics. 2014;7:1-13.
- Gurbuzer B, Pikdöken L, Tunalı M, et al. Scintigraphic evaluation of osteoblastic activity in extraction sockets treated with platelet-rich fibrin. J Oral Maxillofac Surg. 2010;68(5):980-989.
- Hamzacebi B, Oduncuoglo B, Alaaddinogl EE. Treatment of peri-implant bone defects with platelet-rich fibrin. Int J Periodont Restorative Dent. 2015;35(3):415-422.
- Hauser F, Gaydarov N, Badoud I, et al. Clinical and histological evaluation of postextraction platelet-rich fibrin socket filling: a prospective randomized controlled study. Implant Dent. 2013;22(3):295-303.
- Hotwani K, Sharma K. Platelet rich fibrin — a novel acumen into regenerative endodontic therapy. Restor Dent Endod. 2014;39(1):1-6.
- Huang FM, Yang SF, Zhao JH, Chang YC. Platelet-rich fibrin increases proliferation and differentiation of human dental pulp cells. J Endod. 2010;36(10):1628-1632.
- Inchingolo F, Tatullo M, Marrelli M, et al. Trial with Platelet-Rich Fibrin and Bio-Oss used as grafting materials in the treatment of the severe maxillary bone atrophy: clinical and radiological evaluations. Eur Rev Med Pharmacol Sci. 2010;14(12):1075-1084.
- Jain V, Triveni MG, Kumar AB, Mehta DS. Role of platelet-rich fibrin in enhancing palatal wound healing after free graft. Contemp Clin Dent. 2012;3(suppl 2):S240-S243.
- Jankovic S, Aleksic Z, Milinkovic I, Dimitrijevic B. The coronally advanced flap in combination with platelet-rich fibrin (PRF) and enamel matrix derivative in the treatment of gingival recession: a comparative study. Eur J Esthet Dent. 2010;5(3):260-273.
- Jankovic S, Aleksic Z, Klokkevold P, et al. Use of platelet-rich fibrin membrane following treatment of gingival recession: a randomized clinical trial. Int J Periodontics Restor Dent. 2012;32(2):41-50.
- Jayalakshmi KB, Agarwal S, Singh MP, et al. Platelet-rich fibrin with beta-tricalcium phosphate — a novel approach for bone augmentation in chronic periapical lesion: a case report. Case Rep Dent. 2012; doi:10.1155/2012/902858.
- Joseph VR, Raghunath A, Sharma N. Clinical effectiveness of autologous platelet rich fibrin in the management of infrabony periodontal defects. Singapore Dent J. 2012;33(1):5-12.
- Joseph VR, Sam G, Amol NV. Clinical evaluation of autologous platelet rich fibrin in horizontal alveolar bony defects. J Clin Diagn Res. 2014;8(11):ZC43-ZC47
- Kanayama T, Horii K, Senga Y, Shibuya Y. Crestal Approach to Sinus Floor Elevation for Atrophic Maxilla Using Platelet-Rich Fibrin as the Only Grafting Material: A 1-Year Prospective Study. Implant Dent. 2016;25(1):32-38.
- Kathuria A, Chaudhry S, Talwar S, Verma M. Endodontic management of single rooted immature mandibular second molar with single canal using MTA and platelet-rich fibrin membrane barrier: A case report. J Clin Exp Dent. 2011;3(5):e487-e490.
- Kawase T. Platelet-rich plasma and its derivatives as promising bioactive materials for regenerative medicine: basic principles and concepts underlying recent advances. Odontology. 2015;103(2):126-135
- Keceli HG, Kamak G, Erdemir EO, Evginer MS, Dolgun A. The Adjunctive Effect of Platelet-Rich Fibrin to Connective Tissue Graft in the Treatment of Buccal Recession Defects: Results of a Randomized, Parallel-Group Contolled Trial. J Periodontol. 2015;86(11):1221-1230.
- Khetarpal A, Chaudhry S, Talwar S, Verma M. Endodontic management of open apex using MTA and platelet-rich fibrin membrane barrier: A newer matrix concept. J Clin Exp Dent. 2013;5(5):e291-e294.
- Kim JS, Jeong MH, Jo JH, Kim SG, Oh JS. Clinical application of platelet-rich fibrin by the application of the Double J technique during implant placement in alveolar bone defect areas: case reports. Implant Dent. 2013;22(3):244-249.
- Kim JW, Kim SJ, Kim MR. Leucocyte-rich and platelet-rich fibrin for the treatment of bisphosphonate-related osteonecrosis of the jaw: a prospective feasibility study. Br J Oral Maxillofac Surg. 2014;52(9):854-859.
- Krasny K, Kaminski A, Krasny M, et al. Clinical use of allogeneic bone granulates to reconstruct maxillary and mandibular alveolar processes. Transplant Proc. 2011;43(8):3142-3144.
- Kulkarni MR, Thomas BS, Varghese JM, Bhat GS. Platelet-rich fibrin as an adjunct to palatal wound healing after harvesting a free gingival graft: A case series. J Indian Soc Periodontol. 2014;18(3):399-402.
- Kuz’minykh IA. Clinical experience in osteoplastic material Allomatrix-implant and fibrin rich platelets use in surgical treatment of jaw radicular cysts [in Russian]. Stomatologiia (Mosk). 2009;88(1):51-53.
- Lekovic V, Milinkovic I, Aleksic Z, et al. Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects. J Periodontal Res. 2011;47(4):409-417.
- Li Q, Pan S, Dangaria SJ, Gopinathan G, et al. Platelet-rich fibrin promotes periodontal regeneration and enhances alveolar bone augmentation. Biomed Res Int. 2013; doi:10.1155/2013/638043.
- Lundquist R, Dziegiel MH, Ågren MS. Bioactivity and stability of endogenous fibrogenic factors in platelet-rich fibrin. Wound Rep Reg. 2008;16(3):356-363.
- Magremanne M, Baeyens W, Awada S, Vervaet C. Solitary bone cyst of the mandible and platelet rich fibrin (PRF). Rev Stomatol Chir Maxillofac. 2009;110(2):105-108.
- Marenzi G, Riccitiello F, Tia M, di Lauro A, Sammartino G. Influence of Leukocyte- and Platelet-Rich Fibrin (L-PRF) in the Healing of Simple Postextraction Sockets: A Split-Mouth Study. BioMed Res Int. 2015; doi:10.1155/2015/369273.
- Mazor Z, Horowitz RA, Del Corso M, et al. Sinus floor augmentation with simultaneous implant placement using Choukroun’s platelet-rich fibrin as the sole grafting material: a radiologic and histologic study at 6 months. J Periodontol. 2009;80(12):2056-2064.
- Mishra N, Narang I, Mittal N. Platelet-rich fibrin-mediated revitalization of immature necrotic tooth. Contemp Clin Dent. 2013;4(1):412-415.
- Moraschini V, dos Santos EBP. Use of Platelet-Rich Fibrin Membrane in the Treatment of Gingival Recession: a Systematic Review and Meta-Analysis. J Periodontol. 2016;87(3):281-290.
- Nagaveni NB, Poornima P, Joshi JS, Pathak S, Nandini DB. Revascularization of immature, nonvital permanent tooth using platelet-rich fibrin in children. Pediatr Dent. 2015;37(1):1-6.
- Nacopoulos C, Dontas I, Lelovas P, et al. Enhancement of bone regeneration with the combination of platelet-rich fibrin and synthetic graft. J Craniofac Surg. 2014;25(6):2164-2168.
- Nagaveni NB, Kumari KN, Poornima P, Reddy V. Management of an endo-perio lesion in an immature tooth using autologous platelet-rich fibrin: a case report. J Indian Soc Pedod Prev Dent. 2015;33(1):69-73.
- Nørholt SE, Hartlev J. Surgical treatment of osteonecrosis of the jaw with the use of platelet-rich fibrin: a prospective study of 15 patients. Int J Oral Maxillofac Surg. 2016; 45(10):1256-1260.
- Öncü EA, Erbeyoğlu AA. Enhancement of immediate implant stability and recovery by platelet-rich fibrin. Int J Oral Maxillofacial Surg. 2019;39(2)e58-e63.
- Öncu E, Alaaddinoõglu E. Effect of platelet-rich fibrin on osseointegration. Int J Oral Maxillofacial Surg. 2015; 42(10):1265-1266.
- Panda S, Jayakumar ND, Sankari M, Varghese SS, Kumar DS. Platelet rich fibrin and xenograft in treatment of intrabony defect. Contemp Clin Dent. 2014;5(4):550-554.
- Panda S, Ramamoorthi S, Jayakumar ND, Sankari M, Varghese SS. Platelet rich fibrin and alloplast in the treatment of intrabony defect. J Pharm Bioallied Sci. 2014;6(2):127-131.
- Patil PA, Kumar R V, Kripal K. Role and Efficacy of L- PRF matrix in the Regeneration of Periodontal Defect: A New Perspective. J Clin Diagn Res. 2014;8(12):ZD03-ZD05
- Peck MT, Marnewick J, Stephen L. Alveolar ridge preservation using leukocyte and platelet-rich fibrin: a report of a case. Case Rep Dent. 2011; doi:10.1155/2011/345048.
- Pradeep AR, Sharma A. Treatment of 3-wall intrabony defects in chronic periodontitis subjects with autologous platelet rich fibrin: a randomized controlled trial. J Periodontol. 2011;82(12):1705-1712.
- Pradeep AR, Rao NS, Agarwal E, Bajaj P, Kumari M, Naik SB. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of 3-wall intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol. 2012;83(12):1499-1507.
- Pradeep AR, Bajaj P, Rao NS, Agarwal E, Naik SB. Platelet-Rich Fibrin Combined With a Porous Hydroxyapatite Graft for the Treatment of 3-Wall Intrabony Defects in Chronic Periodontitis: A Randomized Controlled Clinical Trial. J Periodontol. 2017;88(12):1288-1296.
- Rajaram V, Thyegarajan R, Balachandran A, Aari G, Kanakamedala A. Platelet Rich Fibrin in double lateral sliding bridge flap procedure for gingival recession coverage: An original study. J Indian Soc Periodontol. 2015;19(6):665-670.
- Ranganathan AT, Chandran CR. Platelet-rich fibrin in the treatment of periodontal bone defects. J Contemp Dent Pract. 2014;15(3):372-375.
- Rao SG, Bhat P, Nagesh KS, et al. Bone Regeneration in Extraction Sockets with Autologous Platelet Rich Fibrin Gel. J Maxillofac Oral Surg. 2013;12(1):11-16.
- Ruga E, Gallesio C, Boffano P. Platelet-rich fibrin and piezoelectric surgery: a safe technique for the prevention of periodontal complications in third molar surgery. J Craniofac Surg. 2011;22(5):1951-1955.
- Sambhav J, Rohit R, Ranjana M, Shalabh M. Platelet rich fibrin (PRF) and B-tricalcium phosphate with coronally advanced flap for the management of Grade II furcation defect. Ethiop J Health Sci. 2014;24(3):269-272.
- Sammartino G, Dohan Ehrenfest DM, Carile F, Tia M, Bucci P (2011). Prevention of hemorrhagic complications after dental extractions into open heart surgery patients under anticoagulant therapy: the use of leukocyte- and platelet-rich fibrin. J Oral Implantol 37: 681-690
- Shah M, Deshpande N, Bharwani A, et al. Effectiveness of autologous platelet-rich fibrin in the treatment of intra-bony defects: A systematic review and meta-analysis. J Indian Soc Periodontol. 2014;18(6):698-704.
- Shah M, Patel J, Dave D, Shah S. Comparative evaluation of platelet-rich fibrin with demineralized freeze-dried bone allograft in periodontal infrabony defects: a randomized controlled clinical study. J Indian Soc Periodontol. 2015;19(1):56-60.
- Sharma A, Pradeep AR. Treatment of 3-wall intrabony defects in patients with chronic periodontitis with autologous platelet-rich fibrin: a randomized controlled clinical trial. J Periodontol. 2011; 82(12):1705-1712.
- Sharma A, Pradeep AR. Autologous platelet-rich fibrin in the treatment of mandibular degree II furcation defects: a randomized clinical trial. J Periodontol. 2011;82(10):1396-1403.
- Shilbli JA, Blay A, Tunchel S, et al. Treatment of peri-implantitis using L-PRF and ER,CR:YSGG laser in the esthetic zone: Longitudinal aspects. In: Rosetti EHO and Bonachela WC, eds, Complex situations on Implant Dentistry: Specialized Clinical Solution. São Paulo, Brazil: VM Vultural; 2013.
- Shivashankar VY, Johns DA, Vidyanath S, Sam G. Combination of platelet rich fibrin, hydroxyapatite and PRF membrane in the management of large inflammatory periapical lesion. J Conserv Dent. 2013;16(3):261-264.
- Shivashankar VY, Johns DA, Vidyanath S, Kumar MR. Platelet Rich Fibrin in the revitalization of tooth with necrotic pulp and open apex. J Conserv Dent. 2012;15(4):395-398.
- Simon BI, Zatcoff AL, Kong JJW, O’Connell SM. Clinical and Histological Comparison of Extraction Socket Healing Following the Use of Autologous Platelet-Rich Fibrin Matrix (PRFM) to Ridge Preservation Procedures Employing Demineralized Freeze Dried Bone Allograft Material and Membrane. Open Dent J. 2009;3:92-99.
- Simonpieri A, Del Corso M, Sammartino G, Dohan Ehrenfest DM. The relevance of Choukroun’s platelet-rich fibrin and metronidazole during complex maxillary rehabilitations using bone allograft. Part I: a new grafting protocol. Implant Dent. 2009;18(2):102-111.
- Simonpieri A, Del Corso M, Sammartino G, Dohan Ehrenfest DM. The relevance of Choukroun’s platelet-rich fibrin and metronidazole during complex maxillary rehabilitations using bone allograft. Part II: implant surgery, prosthodontics, and survival. Implant Dent. 2009;18(3):220-229.
- Simonpieri A, Choukroun J, Del Corso M, Sammartino G, Dohan EDM. Simultaneous sinus-lift and implantation using micro threaded implants and leukocyte- and platelet-rich fibrin as sole grafting material: a six-year experience. Implant Dent. 2011;20(1):2-12.
- Singh A, Kohli M, Nimish Gupta N. Platelet Rich Fibrin: A Novel Approach for Osseous Regeneration. J Maxillofac Oral Surg. 2012;11(4):430-434.
- Singh S, Singh A, Singh S, Singh R. Application of PRF in surgical management of periapical lesions. Natl J Maxillofac Surg. 2013;4(1):94-99.
- Soydan SS, Uckan S. Management of bisphosphonate-related osteonecrosis of the jaw with a platelet-rich fibrin membrane: technical report. J Oral Maxillofac Surg. 2014;72(2):322-326.
- Sundar JS, Varma KM, Kalyan Satish RK, Sajjan GS, Tanikonda R. A Biological Approach in Repair of Damaged Dental Pulp and Periapical Tissues using Platelet Rich Fibrin, Mineral Trioxide Aggregate and Laser Bio stimulation. IJSS Case Reports & Reviews. 2015;1(11):44-50.
- Tajima N, Ohba S, Sawase T, Asahina I. Evaluation of sinus floor augmentation with simultaneous implant placement using platelet- rich fibrin as sole grafting material. Int J Oral Maxillofac Implants. 2013;28:77-83.
- Tatullo M, Marrelli M, Cassetta M, et al. Platelet Rich Fibrin (P.R.F.) in reconstructive surgery of atrophied maxillary bones: clinical and histological evaluations. Int J Med Sci. 2012;9(10):872-880.
- Thorat MK, Pradeep AR, Pallavi B. Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: a controlled clinical trial. J Clin Periodontol. 2011;38(10):925-932.
- Toeroek R, Dohan EDM. The concept of Screw- Guided Bone Regeneration (S-GBR). Part 3: Fast Screw- Guided Bone Regeneration (FS-GBR) in the severely resorbed preimplant posterior mandible using allograft and Leukocyte- and Platelet-Rich Fibrin (L PRF): a 4-year follow-up. POSEIDO. 2013;1(2):93-100.
- Toffler M, Toscano N, Holtzclaw D. Osteotome-mediated sinus floor elevation using only platelet-rich fibrin: an early report on 110 patients. Implant Dent. 2010;19:447-456.
- Triveni MG, Tarun Kumar AB, Vinita Jain, Dhoom S Meh. Alveolar Ridge Preservation with beta-TCP Graft and Platelet-Rich Fibrin. Int J Oral Implant Clin Res. 2012;3:96-100.
- Troiano G, Laino L, Dioguardi M, et al. Mandibular Class II Furcation Defects Treatment: Effects of the Addition of Platelet Concentrates to Open Flap. A Systematic Review and Meta-analysis of RCT. J Periodontol. 2016;87(9):1030-1038.
- Uyanık LO, Bilginaylar K, Etikan I. Effects of platelet- rich fibrin and piezosurgery on impacted mandibular third molar surgery outcomes. Head Face Med. 2015;11:25.
- Vijayalakshmi R, Rajmohan CS, Deepalakshmi D, Sivakami G. Use of platelet rich fibrin in a fenestration defect around an implant. J Indian Soc Periodontol. 2012;16(1):108-112.
- Woo SM, Kim WJ, Lim HS, et al. Combination of Mineral Trioxide Aggregate and Platelet-rich Fibrin Promotes the Odontoblastic Differentiation and Mineralization of Human Dental Pulp Cells via BMP/Smad Signaling Pathway. J Endod. 2016;42(1):82-88.
- Yelamali T, Saikrishna D. Role of Platelet Rich Fibrin and Platelet Rich Plasma in Wound Healing of Extracted Third Molar Sockets: A Comparative Study. J Maxillofac Oral Surg. 2015;14(2):410-416.
- Zhang Y, Tangl S, Huber CD, Lin Y, Qiu L, Rausch-Fan X. Effects of Choukroun’s platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: a histological and histomorphometric study. J Craniomaxillofac Surg. 2012;40(4):321-328.
- Zhao JH, Tsai CH, Chang YC. Clinical and histologic evaluations of healing in an extraction socket filled with platelet-rich fibrin. J Dent Sci. 2011;6(2):116-122.



