Drs. Peter Fairbairn and Sharon Stern present a multi-disciplinary approach to tackling a tricky trauma case
Traumatic injuries to the anterior teeth can be a tragic experience for the patient and require thorough treatment planning, experience, and skill on behalf of the dentist.
Advances in techniques used both in endodontics and implantology have allowed us to save more of the patient’s own teeth — and patients’ wishes to retain their own teeth, if possible, must be respected.
[userloggedin]
In this case study, the use of membrane and autogenous-free bone regeneration with simultaneous implant placement (Fairbairn, 2011; Podaropolous, et al., 2009), as well as microscope-enhanced endodontics, helped achieve the result the patient desired.
Introduction
Dental trauma often involves a team of dental practitioners: the general dentist along with one or more specialist dentists. Since trauma is not a common occurrence in general practice, management of traumatized teeth can be both demanding and challenging, as it is accompanied by emotional factors on the patient’s part.
Horizontal root fractures can be classified according to the location of the fracture line (apical third, middle third, and cervical third). Injury factors to the tooth, such as location of the fracture line, mobility of the coronal fragment, the degree of dislocation of the coronal fragment and diastasis between fragments (rupture of the pulp at the fracture site), stage of root development (immature or mature root), and age of the patient (growth of the alveolar process) have the greatest influence upon healing (Andreasen, et al., 2004; 2007).
In the horizontally fractured tooth, necrosis of the pulp usually occurs in the coronal fragment, while the pulp of the apical fragment remains vital (Andreasen and Hjorting-Hansen 1967; Hitchcock, et al., 1985). This provides a basis for treatment of the horizontally root fractured teeth.
In permanent teeth with horizontal fractures in the apical and middle thirds, root treatment of the coronal fragment only with gutta percha (with calcium hydroxide dressing in the interim) has been proved to be successful, whereas unfavorable outcomes have occurred when both fragments have been endodontically treated with gutta percha (Cvek, et al., 2004; 2008).
The aim of this is to form a calcific barrier at the apical end of the coronal root fragment, in the same way as treating a non-vital immature tooth (by apexification). Mineral trioxide aggregate (MTA), was developed in the 1990s as a root end filling material (Torabinejad, et al., 1993; 1995).
Since then, it has been used extensively in all aspects of endodontic treatment. It is associated with favorable apical healing when used as an apexification material in immature teeth with open apices (Pace, et al., 2007; Simon, et al., 2007; Felippe, et al., 2006) because it encourages hard tissue formation (Pitt Ford, et al., 1996; Nair, et al., 2008; Accorinte Mde, et al., 2008), is biocompatible (Pitt Ford, et al., 1996; Nair, et al., 2008; Aeinechi, et al., 2002), provides a good seal (prevents microleakage) (Torabinejad, et al., 1993; Pitt Ford, et al., 1996; Lee, et al., 1993; Lawley, et al., 2004), and is nonresorbable (Torabinejad and Chivian, 1999). Consequently, MTA is the treatment of choice instead of gutta percha for root filling the coronal segment of teeth with horizontal root fractures.

This case involves three teeth that were involved in trauma and the multi-disciplinary approach used to treat them. After careful assessment, sometimes the only option is removal and replacement with a dental implant. Guided bone regeneration is generally needed in trauma cases where dental implants are to be placed due to bone damage during the trauma or as a result of post-traumatic infection. The co-author has used only alloplast or synthetic particulate graft materials for the last 10 years using no autogenous (blocks, chips, or scrappings) for the last 9 of them. A delayed immediate placement protocol is the standard procedure where the tooth or root is removed carefully, so as to not damage the residual bone, and then left to heal for 3 weeks.
This standard protocol — employed in more than 1,800 cases in the 10 years by the co-author — allows for soft tissue closure yet ensures the preservation of adjacent bone prior to the phase of modeling (Schropp, et al., 2003). Ridge preservation, rather rebuilding the profile of the modeled ridge, can be both more time efficient and less traumatic for the patient. Bone healing is further improved by not using a traditional (collagen-type) membrane that inhibits periosteal blood to the graft site, which accounts for 85% (or more) of the blood supply to the site. The stability and soft tissue cell occlusive properties needed for successful bone regeneration (Schenk 1995) are achieved by a CaSO4 (calcium sulfate) element in the graft material; hence, the graft is its own membrane.
Case
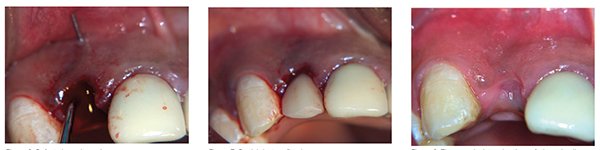
The 25-year-old male patient was involved in a motor vehicle accident that resulted in trauma to his UR1, UR2, and UR3. Horizontal root fractures were evident in the mid to apical third of the UR2 and UR3 (Figure 1). All four teeth were splinted at his local hospital’s dental unit after the initial visit to the accident and emergency (A&E) and later treated by his general dental practitioner.
The case was referred to the authors 3 months post-trauma with a swelling and pain associated with the UR2. Clinical examination revealed that the UR2 was grade 3 mobile; the UR1 and UR3 were firm. The UR3 had not responded to sensitivity tests (electric pulp testing and cold testing). Periapical radiographs of the associated teeth (Figure 2) showed that both the UR2 and UR3 had horizontal root fractures at the junction of the middle and apical third of the roots.
The UR2 was root filled; the coronal fragment was laterally dislocated; the diastasis between the coronal and apical root fragments was over 2 mm; and a lateral radiolucent area was evident. The UR3 was not root filled, the diastasis was less than 1 mm, and lateral radiolucent area was evident. The UR1 was root treated but not ideally obturated; however, no apical radiolucencies were associated with these roots.
The patient was determined to retain both the UR2 and UR3. Since the 13 was not mobile, the diastasis between the coronal and apical fragments was less than 1 mm and had no associated pockets, the prognosis for treating this tooth was good.
However, the fact that the UR2 had grade 3 mobility, the only option for the lateral incisor was an extraction. This prospect suited the patient who had been initially referred for the placement of two implants, and the necessary treatment consent was completed. The initial treatment would be to secure the future of the canine, and endodontic treatment was arranged.
Endodontic treatment of the UR3
A decision was made to treat only the coronal fragment of the UR3 as the apical fragment was assumed to be vital (Andreasen and Hjorting-Hansen 1967; Hitchcock, et al., 1985). Rubber dam was secured over the tooth using a Q9 rubber dam clamp (Dentsply Ash instruments, UK). The access was established with a long tapered diamond bur. The pulp chamber was then fully accessed and refined using a BUC-1 ultrasonic tip under the copious water spray. One canal was identified with the aid of an operating microscope (Global G3, Global Surgical Corporation) using a DG16 explorer probe (Dentsply Ash instruments).
The working length of the root canal of the coronal fragment was determined using an apex locator (Raypex® 5; VDW). A working length radiograph was taken to verify the apex locator readings . The canals were instrumented to working length with hand K-Flexofiles® (Dentsply Maillefer) to an ISO size 70 using the balanced force technique.
The UR3 was root filled to the level of the root fracture with a minimum of 4 mm of mineral trioxide aggregate (MTA) (Angelus) using the Messing Root Canal Gun (Miltex) to deliver the MTA (Figure 3). An activated, stainless steel ultrasonic tip was used to apply ultrasonic energy to a number 2/3 Machtou condenser (Dentsply Maillefer), which was used to pack, flow, and settle the MTA. The rest of the root canal was backfilled with gutta percha, and the access cavity was restored with composite (Filtek™ Supreme XT Universal Composite, 3M ESPE). A postoperative radiograph of the completed root canal treatment was taken (Figure 4). There is slight extrusion of the MTA beyond the fracture line; however, since MTA is biocompatible, the prognosis of the treatment is still good.
Implant placement at UR2
The surgical phase was then initiated with the removal of the fractured lateral incisor. A plastic partial denture was made as a temporary rather than the preferred resin-bonded bridge due to cost factors and the patient’s desire not to involve adjacent teeth.


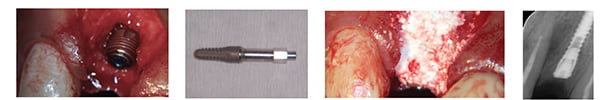
The root tip was removed using a Periotome (Figures 5A and 5B), taking care not to damage the buccal plate any further. Probing the socket showed the resultant buccal bone defect (Figure 6) and the thin biotype of the gingiva. The partial denture was then fitted (Figure 7), and the site was then allowed to heal for 3 weeks.
After the period of soft tissue healing, we generally have good enough soft tissue closure (Figures 8 and 9), but the effects of hard tissue modeling can already be seen due to the extent of the infected site bone loss. A site-specific flap is then raised not to affect the papillae of the adjacent teeth.
The concept of employing the membrane in the graft (Fairbairn 2011; Podaropolous, et al., 2009) allows this flap to be smaller, reducing patient trauma, as well as allowing the all-important blood supply from the periosteum unimpeded access to the site. The periosteum in a bone damage site also plays a role in the induction of stromal cell derived factors (Fairbairn 2011), which results in an increased presence of mesenchymal cells important for healing (Zhao, et al., 2012). Thus, the author feels the use of traditional collagen-type membranes may be a hindrance rather than a help to the body’s healing (Gutta, et al., 2009).
The site was then vigorously curetted to ensure the removal of any granulation tissue. The bacteriostatic nature of CaSO4 enabled the co-author to dispense with the need for the use of chlorhexidine, even though its effect on fibroblasts is debatable.
A DIO 3.8 mm by 12 mm implant (DIO Implant Corporation) was placed slightly palatally in the socket (Figures 10-11) to the desired torque of 35 Nm. The author always places the implant at the time of grafting — even in extreme bone loss cases — due to the inherent regenerative capabilities of the titanium implant (Brunette 2001), as well as its mechanical stabilization of the particulate graft.
The implant can thus be considered the most important of graft materials — as well as aiding the bone regeneration, it will be needed to attach the abutment and crown in the near future.
The Osstell reading (Bornstein, et al., ND) was then taken using a type 49 peg, which here was 38 ISQ, a low reading. Always make sure to correctly seat the peg as shown (Figure 12) to prevent incorrect readings. The particulate graft (Vital, Biocomposites) was prepared according to the manufacturer’s instructions and packed into the site and allowed to “set” using gauze to restrict the blood ingression into the site for a 3-minute period (Figure 13). This ability to set and hence become more stable has been shown to lead to more successful graft site with improved bone regeneration (Schenk 1995). The site was then closed carefully and sutured using 5.0 Vicryl™ sutures (Ethicon, Inc. [Figure 14]).
The CaSO4 element of the material will supply a soft tissue cell occlusive barrier for the first 3 weeks (patient dependent) while being vascularly porous to ensure angiogenesis. This vascular porosity increases as the CaSO4 element bio-absorbs, providing elements for the bone regeneration process in the structure of the BTcP (99% pure beta tri-calcium phosphate) element of the material (Smeets, et al., 2009).
It is also noted that most particulate graft materials (Vital in particular) exhibit a negative iso-electric charge in an aqueous solution, which attracts host bone morphogenetic proteins (BMPs) such as osteoponin and osteocalcin in greater numbers to the site (Hunt and Cooper 2012). These then attract the host’s negatively charged mesenchymal cells (osteoblasts) and therefore up-regulating the host healing response. Hence, the author has not used any autogenous bone in the last 9 years as he feels introducing dead bone to the site delays the healing process due to the initial osteoclastic phase.
Not using autogenous bone results in reduced patient morbidity and hence a greater acceptance of the surgical procedures. After 12 weeks, a flap was then raised to show new bone formation, with some remnant graft material on the surface (Figure 15). A round bur (Meisinger) was used to access the implant head completely (Figure 16), which is important to seat the Osstell peg perfectly and prevent false readings.
The full bio-absorption of the graft material is important in returning the site to true human host bone. Numerous research papers by the co-author (Leventis, et al., 2012) and others have shown that by 10 weeks up to 85% of the graft material may have already bio-absorbed to facilitate improved bone regeneration in line with the host healing process (Figure 17).
The flap was also used to move the attached, keratinized gingival tissue buccally (a small rollover type flap) when the healing cap (SANH 4224) was fitted and the denture re-fitted for another week (Figure 18).

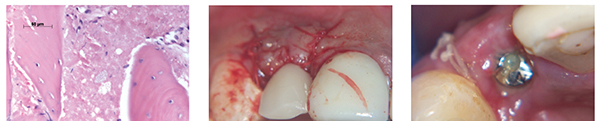

The further improvement in the profile can be seen in Figure 19. The correct abutment (SACN 4835T) was selected to move the crown margin to a level 1 mm below the gingival margin despite the deeper placement of the implant (Figure 20). This was used to optimize the platform switching benefits of this indexed tapered abutment system and improve the soft tissue seal above the implant.
An IPS e.max® crown (Ivoclar Vivadent) was made and cemented with Premier® Implant Cement (Premier Products Co.) The excess was removed during the “gel” phase to ensure no residual cement was left sub-gingivally. The patient was happy with the outcome and was asked attend every 6 months to enable long-term assessment of this more complicated case.
Review
At the first review appointment, an improved buccal profile and gingival health was observed (Figure 21) with stippling and no bleeding on probing despite the excess cement of a recently re-cemented veneer on the UR1.
Radiographically, the bone density appeared to have improved in the cervical area (Figure 22), possibly as a result of functional remodeling and the final “turning over” of the remnant graft material, which can take up to 9 months depending on patient physiology.

This full bio-absorption of the graft material is important to return the host bone back to a healthy state without the presence of foreign hydroxyapatite (HA), which may impede the natural osteoclastic and osteoblastic cycle of natural bone. Once loaded, there appears to be little change in the profile in line with Wolff’s Law, in that function is essential to retain bone.
Twelve months after the UR3 was root treated, the lateral radiolucent area associated with the UR3 shows bone remodeling (Figure 23). It can take up to 4 years for healing to occur fully (Torabinejad and Chivian 1999). The patient has been symptom free, the UR3 is not mobile, no pockets over 3 mm are evident, and there are no swellings or sinus tracts present. The overall prognosis for the UR3 is good.
Discussion
At 1 year following loading, patient recall showed further bone regeneration in the UR2 area due to further functional remodelling (Figure 23). The co-author feels the need for the use of a particulate graft material in the repair of bone defects — not only to provide a scaffold for the bone regeneration but also for the up-regulation of the host response, with their use as shown in recent research that tested 38,000 genes (Zhao, et al., 2012).
The patient’s oral hygiene was not ideal due to a reluctance to floss, but again no bleeding on probing was observed, and the patient had no adverse symptoms from the treatment. Healthy papillae were retained (Figure 24), although the need for improved OH was again stressed.
The prognosis of root fractured teeth depends on the extent of the fracture line, the pulp tissue status, mobility of the coronal fragment, and dislocation of fragments (Andreasen, et al., 2004). Survival is poorest for root fractures located at the gingival third of the root (Welbury, et al., 2002).
The UR3 was horizontally fractured at the junction of the middle and apical third of the roots. It was not mobile, and the coronal fragments did not appear dislocated; hence, the prognosis for treatment was good.
The International Association of Dental Traumatology (IADT) guidelines (Flores, et al., 2007) recommend endodontic treatment only after pulp necrosis, not as a prophylactic intervention. Trauma cases should be carefully monitored clinically, radiographically, and with sensitivity tests (thermal, electric pulp testing). The treating practitioner should treat each case individually as no trauma case is the same.
In this case pulpal necrosis developed, and endodontic treatment of the coronal fragment only was indicated, as root fractured teeth often possess a vital apical fragment even when the coronal fragment is necrotic (Andreasen and Hjorting-Hansen 1967; Hitchcock, et al., 1985; Cvek, et al., 2004).
In the study by Cvek, et al., (2004), gutta percha was used to fill the root canal, and the authors found that overfilled root canal filling material between the fragments did not lead to healing. In this case healing was evident even though the root canal was overfilled; this could be because MTA was used instead of gutta percha.
Radiological evaluation of root fractures is usually based on multiple periapical radiographs and occlusal views; however, with cone beam CT (the patient declined this), it is possible to examine the root in three dimensions, and this may aid in further assessment of the prognosis of the injured tooth.
Conclusion
The result achieved for the patient has exceeded his expectations, with the use of newer materials and techniques having reduced both the treatment time scale as well as patient morbidity.
These synthetic bone regeneration materials also negate the need for a material specific consent procedure, and their ability to “turnover” to host bone is often a vital factor in the patient consenting to the entire treatment plan as no remnant donor material (human or bovine) is present in years to come.
Material and technique advances in endondontics have also allowed us to treat fractured roots, providing the correct protocols are initially followed.
Accordingly, the patient’s desire for a cost-effective, low-pain, and ethical solution have been met.
[/userloggedin]
[userloggedout][/userloggedout]
Accorinte Mde L, Holland R, Reis A, Bortoluzzi MC, Murata SS, Dezan E Jr, Souza V, Alessandro LD. Evaluation of mineral trioxide aggregate and calcium hydroxide cement as pulp-capping agents in human teeth. J Endod. 2008;34:1-6.
Aeinechi M, Eslami B, Ghanbariha M, Saffar AS. Mineral trioxide aggregate and calcium hydroxide as pulp capping agents in human teeth: A preliminary report. Int Endod J. 2002;36:225-31.
Andreasen FM, Andreasen JO, Cvek M. Root fractures. In: Andreasen Jo, Andreasen FM, Andersson L(eds.):Textbook and color atlas of traumatic injuries to the teeth. Munksgaard, Kopenhagen. Wiley-Blackwell, 2007:337-371.
Andreasen JO, Andreasen FM, Mejare I, Cvek M. Healing of 400 intra-alveolar root-fractures: 1. Effect of pre-injury and injury factors such as sex, age, stage of root development, fracture type, location of fracture and severity of dislocation. Dental Traumatol. 2004;20:192-202.
Andreasen JO, Hjorting-Hansen E.Intraalveolar root fractures, radiographic and histology study of 50 cases. J Oral Surg. 1967;25:414-26.
Bornstein M, Hart C, Buser D et al.,. Early loading of nonsubmerged Titanium Implants with a chemically modified, sandblasted and acid-etched surface ; 6 month results of a prospective case series study in the posterior mandible, focusing on peri-implant crestal bone changes and Implant stability Quotient (ISQ) values. Clin Implant Dent Relat Res. 2009;11(4):338-47.
Brunette TM (2001). Titanium in Medicine. Springer 649-673.
Cvek M, Mejare I, Andreasen JO. Conservative treatment of teeth fractured in the middle or apical part of the root. Dent Traumatol. 2004;20:261-269.
Cvek M, Tsilingaridis G, Andreasen JO. Survival of 534 incisors after intra-alveolar root fracture in patients aged 7-17 years. Dental Traumatol. 2008;24:379-87.
Fairbairn P. Membrane-free guided bone regeneration. EDI Journal. 2011;7(3):74-80.
Felippe WT, Felippe MC, Rocha MJ. The effect of mineral trioxide aggregate on the apexification and periapical healing of teeth with incomplete root formation. Int Endod J. 2006;39:2-9.
Flores MT, Andersson L, Andreasen JO, Bakland LK, Malmgren B, Barnett F, et al.,. International Association of Dental Traumatology. Guidelines for the management of traumatic dental injuries. I. Fractures and luxations of permanent teeth. Dent Traumatol. 2007;23:66-71.
Gutta R, Baker R, et al.,. Barrier membranes used for ridge augmentation: is there an optimal pore size? J Oral Maxillofac Surg. 2009;67(6):1218-25.
Hitchcock R, Ellis E, Cox CF. Intentional vital root resection: a 52 week histopathologic study in Macaca Mulatta. J Oral Surg., Oral Med., Oral Path. 1985;60: 2-14.
Hunt JA, Cooper JJ (2012). The significance of zeta potential in osteogenesis. society for biomaterials , 31st meeting for biomaterials , Pittsburgh, PA 2006. 2012;592.
Lawley GR, Schindler WG, Walker WA, Kolodrubetz D. Evaluation of ultrasonically placed MTA and fracture resistance with intracanal composite resin in a model of apexification. J Endod. 2004;30:167-172.
Lee SJ, Monsef M, Torabinejad M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations.0 1993;19:541-4.
Leventis M, Fairbairn P, Vasiliadis O et al.,. Socket Grafting using Beta Tri-Calcium Phosphate in a Calcium Sulfate matrix. EAO Poster, Copenhagen. 2012: 525.
Nair PN, Duncan HF, Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. Int Endod J. 2008;41:128-150.
Pace R, Giuliani V, Pin Prato L, Baccetti T, Pagavino G. Apical plug technique using mineral trioxide aggregate: results from a case series. Int Endod J. 2007; 40:478-484.
Pitt Ford TR, Torabinejad M, Abedi HR, Bakland LK, Kariyawasam SP. Using mineral trioxide aggregate as a pulp-capping material. J Am Dent Assoc. 1996; 127: 1491-4.
Podaropolous L, Vies A et al.,. Bone regeneration using Beta Tri-Calcium Phosphate in a Calcium Sulfate matrix, Journal of Oral Implantology 35(1):28-36.
Schenk RK. Bone regeneration: biologic basis. In: Buser D, Dahlin K, and Schenk RK, eds. Guided Bone regeneration in Implant Dentistry. London, UK: Quintessence; 1995:49-100.
Schropp L, Wenzel A, et al.,. Bone Healing and soft tissue changes following a single tooth extraction: A Clinical and radiographic 12 month study. Int J Periodontics Restorative Dent. 2003;23(4):313-23.
Simon S, Rilliard F, Berdal A, Machtou P. The use of mineral trioxide aggregate in one visit apexification treatment: a prospective study. Int Endod J. 2007;40:186-197.
Smeets R, Kolk A, et al., A new biphasic osteoinductive calcium composite material with a negative zeta potential for bone augmentation. Head & Face Medicine. 2009;13(5):13.
Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25:197-205.
Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21:349-353.
Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root-end filling material. J Endod. 1995; 19:591-595.
Welbury RR, Kinirons MJ , Day P, Humphreys K, Gregg TA. Outcomes for root-fractured permanent incisors: a retrospective study. Pediatr Dent. 2002;24: 98-102.
Zhao J, Watanabe T et al.,. Transcriptome analysis of BTcP implanted in a dog mandible bone. Elsevier. 2012;864-877.
Stay Relevant With Implant Practice US
Join our email list for CE courses and webinars, articles and mores



