 Albuquerque, New Mexico, August 3, 2015, OCO Biomedical, Inc. – OCO Biomedical, Inc., a proven global leader in world-class dental technology, training and instrumentation, has announced that Dentistry Today, one of the nation’s leading clinical news magazines for dentists, has listed OCO’s one-piece, small diameter implant (SDI) line as one of the this year’s Top 100 Products in their July, 2015, issue.
Albuquerque, New Mexico, August 3, 2015, OCO Biomedical, Inc. – OCO Biomedical, Inc., a proven global leader in world-class dental technology, training and instrumentation, has announced that Dentistry Today, one of the nation’s leading clinical news magazines for dentists, has listed OCO’s one-piece, small diameter implant (SDI) line as one of the this year’s Top 100 Products in their July, 2015, issue.
According to the company, OCO’s clinically proven, value-priced SDI line is known for versatility, high-performance, innovation in design and increased initial stability. The SDI line has also been well-received at prestigious international and national trade shows such as the International Dental Show (IDS) in Cologne, Germany, The Dentistry Show in Birmingham, UK, AAID, GNYDM and numerous other meetings and exhibitions held across the USA.

States Dr. David D. Dalise, OCO Biomedical Founder and Chief Executive Officer, “Since first being introduced, and after personally placing and testing thousands of our one-piece SDI products, the line has continued to gain acceptance and popularity with practitioners world-wide as an affordable alternative to full-size implants, particularly as a treatment option for thin ridge patients. We truly are proud, year after year, to design award-winning products by dentists for dentists that improve patient care and build practice performance.”
OCO Biomedical SDI Features and Benefits include:
- Diameters: Available in 2.2, 2.5 and 2.9mm
- Lengths: 10, 12, 14 and 16mm
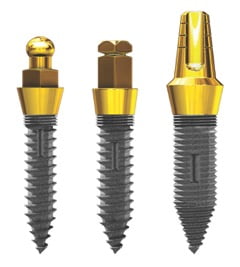 Clinically-proven and precision machined out of Grade 23 ELI titanium alloy
Clinically-proven and precision machined out of Grade 23 ELI titanium alloy- Designed with a machined not a polished collar and engineered with an aggressive thread pattern for increased initial stability
- Threads finished with OCO’s propriety SLA surface treatment
- Compatible with competitor’s wrenches and drivers (2.2 and 2.5mm only)
- Perfect mini alternative, without costly investment for new instrumentation
- Logical progression to placing OCO’s larger diameter one-piece implants: the 3.0mm and ISI; as well as the TSI and ERI and ENGAGE two-stage implants
- Manufactured in the United States
For further information about OCO’s extensive product line, course schedules for Complete Dental Implant Solutions Approach training and registration for the 2016 OCO Biomedical International Dental Implant Symposium, call OCO Biomedical at 1-800-228-0477 or visit www.ocobiomedical.com Print product catalogs are available upon request.
About OCO Biomedical, Inc.
Established in 1977 and headquartered in Albuquerque, New Mexico, OCO Biomedical, Inc. is a privately-owned dental implant company. In addition to the Company’s vast network of practitioners using OCO products in the United States, the Company has an international network of distributors located in Asia, Central and South America, Europe and the Caribbean. OCO Biomedical, Inc. is a proven world leader in creating and supplying patented, brand-name dental implant products, technology and AGD-Pace CE accredited education and training in North America. OCO Biomedical, Inc. is the implant company that provides complete implant solutions allowing practitioners to effectively serve their patients while simultaneously building practice performance.
Stay Relevant With Implant Practice US
Join our email list for CE courses and webinars, articles and mores



